
Research Article
Austin J Clin Pathol. 2021; 8(1): 1071.
Effects of Glucan Supplementation on Aged Hematopoietic Progenitor Cells
Vetvicka V* and Vetvickova J
Department of Pathology, University of Louisville, USA
*Corresponding author: Vaclav Vetvicka, Department of Pathology, University of Louisville, 511 S. Floyd, Louisville, KY 40202, USA
Received: June 01, 2021; Accepted: June 23, 2021; Published: June 30, 2021
Abstract
Age related changes in activity of hematopoietic progenitor cells led us to investigate the possibility that glucan supplementation might overcome this suppression. In our study, we focused on differences of these cells in young (8 weeks old) and old (18 months old) mice after supplementation of yeast-derived insoluble glucan. Our results showed significant differences in reconstitution ability of progenitor cells as well as changes in level of stromal cell-derived factor 1α between young and old mice exist and can be partly restored by glucan supplementation.
Keywords: Glucan; Progenitor cells; Aging; Supplementation
Introduction
β1,3 Glucans (glucan further on) are structurally complex homopolymers of glucose, usually isolated from yeast and fungi. Glucans belong to a group of natural, physiologically active compounds, generally called biological response modifiers. Glucan’s role as a biologically active immunomodulator has been well documented for more than 60 years. Interest in the immunomodulatory properties of polysaccharides was initially raised after experiments showing that a crude yeast cell preparation stimulated macrophages via activation of the complement system [1]. Further work identified the immunomodulatory active component as β1,3-glucan [2]. Glucans show notable physiological effects; this is their most important quality and the reason why so much attention has been focused on them. Numerous studies (currently more than 25,000 publications) have subsequently shown that glucans, either particulate or soluble, exhibit immunostimulating properties, including antibacterial and anti-tumor activities [3,4]. The original studies of effects of glucan on the immune system focused entirely on mice. Later, additional studies demonstrated that glucan possesses a significant immunostimulating activity in a wide variety of species including earthworms, bees, shrimp, fish, chicken, rats, rabbits, guinea pigs, sheep, pigs, cattle, and, of course, humans. These results led to a conclusion that β-glucan represents a unique type of immunostimulating molecule that is actively spanning full evolutionary spectrum [5]. However, despite decades of intensive research, the mechanisms of how glucan affected, our defense reactions are still not fully explained [6-8], clearly showing the need for more research.
Despite intensive research, particularly in last two decades, very little is done on possible effect of glucan on aged animals or on hematopoiesis. Mostly, glucan has been found to support hematopoiesis experimentally suppressed by chemotherapy or radiation [9]. More study that is detailed found that glucan supports mobilization of hematopoietic progenitor cells via activation of matrix metalloproteinase-9 [10]. In our study, we focused on possible differences in glucan action in young and old mice.
Materials and Methods
Animals
Female, 8 week old (young) and 18 months old (old) BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The University of Louisville IACUC Committee has approved the protocol for the research project and it conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). Animals were sacrificed by CO2 asphyxiation followed by cervical dislocation.
Glucan
Yeast-derived insoluble Glucan #300 was purchased from Transfer Point (Columbia, SC). The purity is over 85%. An oral or i.v. dose of 100μg/mouse was used.
Materials
Ficoll-Hypaque and M-199 medium were purchased from Sigma (St. Louis, USA), GM-CSF from (StemCell Technologies (Vancouver, Canada), and IL-3 from Cell Sciences (Canton, USA).
HPC mobilization
Mice were given a single i.v. injection (via tail vein) of glucan. Peripheral blood was collected by cardiac puncture at the indicated time. HPC mobilization was evaluated as described earlier [10] by methycellulose culture assay. Blood was diluted with 5ml of PBS and underlayed with 2ml of Ficoll-Hypaque. After centrifugation, the leukocyte layer was removed, washed and resuspended in 2ml of M-199 medium. Cell suspension (4x105) was mixed with 4ml of MethoCult medium containing 5ng/ml recombinant mouse GMCSF and 5ng/ml mouse IL-3. Cells were plated at 2x105 cells/ml in 6-well tissue culture plates and incubated at 370C for 6 days.
ELISA
Mouse stromal cell-derived factor 1α levels were measured by ELISA (Quantikine, R&D Systems) according to manufacturer’s instruction.
Reconstitution
Male mice were treated with glucan as a single i.v. dose. Peripheral blood was collected and single-cell suspension prepared. In separate experiments, cells were injected i.v. into irradiated female mice to assay their long-term (three months) in vivo reconstitution potential. Female recipients were exposed to 0.9Gy/min using Gammacell-40 source (Atomic Energy, Ottawa, Canada). Reconstitutional analysis was done as previously described (Patchen et al, 1998). Briefly, the femur, spleen and thymus were collected and DNAextracted to prepare Southern blots. Equal amount of DNA from each sample was loaded per gel and DNA analyzed for presence of male Y-specific sequence. Blots were probed overnight at 650C and autoradiography was performed at -700C. Filters were then exposed to photostimulatable storage phosphoimaging plates (Molecular Dynamics, Synnyvale, USA) and percent of male DNA in each sample was quantified based on male murine DNA standard.
Statistical analysis
Student’s unpaired t-test was used to calculate statistical differences between data sets. Significant difference was taken at p <0.05.
Results
Stromal Cell-Derived Factor 1α (SDF-1α), also known as CXCL12, is an important chemoattractant. Its blood levels significantly dropped in aged mice. As shown in Table 1, glucan supplementation significantly increased SDF-1α levels in both young and old mice. In old mice, the glucan-stimulated group showed the same SDF-1α level as untreated young mice.
Young
Old
PBS
2.34 ± 0.42*,**
0.57 ± 0.22
Glucan
4.79 ± 0.43*,**
2.12 ± 0.51
Results given as mean ng/ml values ± SD. *Significant difference between glucan and PBS groups at P<0.05 level, **Significant differences between young and old mice at P<0.05 level.
Table 1: Stromal cell-derived factor 1a levels in plasma.
To determine the ability of glucan to mobilize hematopoietic progenitor cells to the periphery, the time kinetics of this mobilization with a single dose of glucan were tested. After an i.v. application of glucan, CFUs were cultured from peripheral blood collected at time intervals shown in Figure 1. On both cases, the peak CFU mobilization was found at 24 hrs after glucan application. The effects lasted until 48hrs and then started to decline (data not shown). With respect of different age groups, the glucan’s effects were more pronounced in young mice in 1 to 4 hrs times, but identical at the peak.
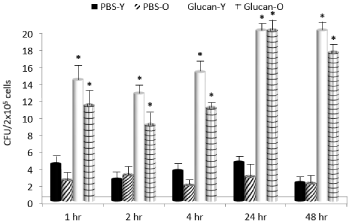
Figure 1: Glucan-mediated mobilization of hematopoietic progenitor cells.
Time kinetics after a single i.v. dose of glucan. *Significant differences
between glucan and PBS groups at P<0.05 level.
In next part of the study, we focused on bone marrow, spleen and thymus reconstitution. All mice transplanted with cells at this dose survived until euthanized to assay the reconstitutional potential of cells based on their ability to repopulate the bone marrow, spleen and thymus of the female recipients. The enhanced reconstitution observed with glucan-mobilized cells was significantly more pronounced in young animals. In control mice, the mobilization was also lower in old mice (Figure 2-4). When compared to mobilization with G-CSF, glucan was similarly active (data not shown).
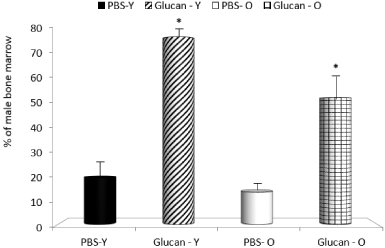
Figure 2: Long-term bone marrow reconstitution produced by mobilized
peripheral blood cells. *Significant differences between glucan and PBS
groups at P<0.05 level.
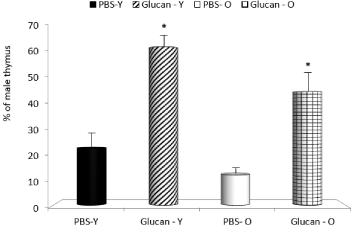
Figure 3: Long-term thymus reconstitution produced by mobilized peripheral
blood cells. *Significant differences between glucan and PBS groups at
P<0.05 level.
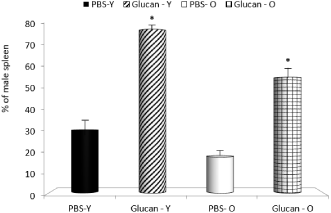
Figure 4: Long-term spleen reconstitution produced by mobilized peripheral
blood cells. *Significant differences between glucan and PBS groups at
P<0.05 level.
Discussion
Mature blood cells have a finite lifespan and need to be continuously replaced throughout life. These cells originate from a small population of pluripotent hematopoietic stem cells. These cells, however, undergo profound changes with age accompanied with blunted responsiveness to tissue injury, proliferative capacities and declining functional activities, resulting in gradual loss of tissue homeostasis and immune dysfunction [11]. Therefore, the reversal of stem cell aging at the cellular level might be highly important in helping the organism to overcome decline of the defense reactions and some diseases connected with the activity of immune system. One of the rare comparisons of hematopoietic stem cells in young and old mice showed that the numbers of these cells are comparable, but these cells obtained from old animals were only about 25% as efficient at homing to and engrafting the bone marrow of irradiated mice [12]. Additional report showed significant effects of aging on hematopoietic progenitor cells [13]. From these studies, however, was not clear if these changes are intrinsic or caused by the aging of their environment. Sporadic studies on possible effects of glucan on progenitor cells suggested positive outcome of glucan supplementation. An interesting study found that yeast-derived glucan will enhance hematopoietic progenitor cell mobilization and might improve the outcome of stem cell transplantation in clinical practice [10].
Beta glucans are structural components of cell walls in many different sources including bacteria, fungi, algae and yeast. Botanical polysaccharides are gaining increased interest in biomedical research due to their broad spectrum of therapeutic properties, together with relatively low toxicity. Glucans have also been studied as an alternative to antibiotics in food animal production, including poultry, fish and pigs. Glucans are considered strong activators of cellular immunity, with macrophages being the most important targets. Based on the first wave of studies, glucan has been established in protection against infection. The protective effects of glucan application were shown in experimental models of infection with Leishmania major, Candida albicans, Toxoplasma gondii, Streptococcus suis, Staphylococcus aureus, Escherichia coli, Mesocestoides corti, Trypanosoma cruzi, and Bacillus anthracis. The attention of scientists focused on the fight against cancer. Since the original study published more than 35 years ago, the antitumor activity of glucan has been published. In fact, numerous animal and human studies have shown remarkable activity against a wide variety of tumors. Further, studies that are more recent have repeatedly shown that glucan has a strong synergy with the antibodies, which naturally occur in cancer [14]. With such pleiotropic effects, it is not surprising that glucan was also tested for possible effects on hematopoietic cells.
First, we focused on the differences in serum levels of SDF-1a, which is also known as CXCL12, which is an important chemokine involved in numerous biological and pathological processes including angiogenesis and hematopoiesis. It is responsible for the retention of hematopoietic progenitor and stem cells [15]. It is clear that level of this chemokine is strongly suppressed in old animals and glucan supplementation can restore it.
Long-term effects of bone marrow, thymus and spleen reconstitution produced by mobilized peripheral blood cells showed again that glucan supplementation improves age-related depression of progenitor cells. These data agree with some previously described results obtained by using soluble glucan [16].
To date, no mechanisms has been elucidated to define how glucan mobilizes progenitor cells. It is hypothesized that upon binding to specific receptors (most of all CR3) on stem cells, which subsequently results in accelerated hematopoietic recovery, most probably via increased proliferation of progenitor cells [17]. Similar mechanism can be expected in our study, as the expression of CR3 changes with age [18]. From our data, we can conclude that the age-related decrease in hematopoietic progenitor functions can be at least partly reduced by glucan supplementation. We hypothesize that glucan supplementation might be developed into a new rejuvenating strategy for healthy aging [11].
Author’s Contribution
VV designed the study, carried out the analysis, interpreted the data, and drafted the manuscript. JV performed in vitro experiments and performed data analysis. Both authors have read, edited and approved the final manuscript.
References
- Benacerraf B, Sebestyen MM. Effect of bacterial endotoxins on the reticuloendothelial system. Fed. Proc. 1957; 16: 860-867.
- Rigi SJ, Di Luzio NR. Identification of a reticuloendothelial-stimulating agent in zymosan. Am. J. Physiol. 1961; 200: 297-300.
- Novak M, Vetvicka V. Beta-glucans, history and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008; 5: 47-57.
- Novak M, Vetvicka V. Glucans as biological response modifiers. Endocrine Metabol. Immune Disorders-Drug Targets. 2009; 9: 67-75.
- Vetvicka V, Sima P. β-Glucan in invertebrates. Invertebrate Survival J. 2004; 1: 60-65.
- Clark AE, Kerrigan AM, Brown GD. β-Glucan receptors, in Vetvicka V, Novak M (eds), Biology and Chemistry of Beta Glucan, Bentham Science. 2001; 39-48.
- Drummond RA, Brown GD. The role of Dectin-1 in the host defense against fungal infections. Curr Opin Microbiol. 2011; 14: 392-399.
- Williams DL, Ha T, Li C, Laffan J, Kalbfleisch J, Laffan JJ, et al. Inhibition of LPS-induced NFkappaβ activation by a glucan ligand involves downregulation of IKKbeta kinase activity and altered phosphorylation and degradation of Ikappaβalpha. Shock. 2000; 13: 446-452.
- Hoffer M, Pospisil M. Modulation of animal and human hematopoiesis by β-glucans: A review. Molecules. 2011; 16: 7969-7979.
- Cramer DE, Wagner S, Li B, Liu JJ, Hansen R, Reca R, et al. Mobilization of hematopoietic progenitor cells by yeast-derived β-glucan requires activation of matrix-metalloproteinase-9. Stem Cells. 2008; 26: 1231-1240.
- Wang M, Chen JJ, Chen F, Liu Q, Sun Y, Yan C, et al. Rejuvenating strategies of tissue-specific stem cells for healthy aging. Aging Dis. 2019; 10: 871-882.
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weismann IL. The aging of hematopoietic stem cells. Nature Med. 1996; 2: 1011-1016.
- Waterstrat A, Van Zant G. Effect of aging on hematopoietic stem and progenitor cells. Curr. Opin. Immunol. 2009; 21: 408-413.
- Vetvicka V, Vannucci L, Sima P, Richter J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019; 24: 1251.
- Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018; 15: 299-311.
- Patchen ML, Liang J, Vaudrain T, Martin T, Melican D, Zhong S, et al. Mobilization of peripheral blood progenitor cells by Betafection PGG-glucan alone and in combination with granulocyte colony-stimulating factor. Stem Cells. 1998; 16: 208-217.
- Cramer DE, Allendorf DJ, Baran JAT, Hansen R, Marroquin J, Li B, et al. Beta-glucan enhances complement-mediated hematopoietic recovery after bone marrow injury. Blood. 2006; 107: 835-840.
- Smith JB, Campbell DE, Ludomirsky A, Polin ARA, Douglas SD, Garty BZ, et al. Expression of the complement receptors CR1 and CR3 and the type III Fc gamma receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediat. Res. 1990; 28: 120-126.