
Review Article
Austin J Clin Pathol. 2021; 8(2): 1073.
Structural Changes in the Lung during Covid-19 Infection and Its Effect on Mortality Rate and Prognosis
Kasem MS¹, El-Khsosy AAF¹, Sherif WS¹*, Rashad NZ¹, Abdelazeem NH¹, Teima ME¹, Sharaf WK¹, Shehata AW¹ and Domouky AM²
¹Faculty of Medicine, Zagazig University, Egypt
²Human Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt
*Corresponding author: Sherif WS, Third Year Medical Student, Faculty of Medicine, Zagazig University, 44519, Egypt
Received: September 13, 2021; Accepted: October 09, 2021;Published: October 16, 2021
Abstract
COVID-19 or SARS-Cov-19 has emerged in late 2019 as one of the highly pathogenic transmissible viruses ever known, comprising many features regarding its transmission and manifestations similar to those of its older siblings, SARS and MERS. COVID-19 has caused a pandemic that forced most of the world into isolation after threatening the lives of millions. In this article we shed more light on the structural changes that happens to the lung in the context of Reverse Transcription-Polymerase Chain Reaction (RT-PCR) confirmed COVID-19 infection based on gross examinations and CT scans, moreover, to correlate these changes to mortality rate hoping to identify the prognostic factors by which prognosis of the disease and mortality rate could be expected, higher involvement with higher severity degree in a short span of time indicates poor prognosis and high mortality chance, reviewing the literature data and records showed that the lungs undergo many changes on both macroscopic and microscopic levels (e.g. ground-glass opacity) which relates to the pathogenesis of the disease and manifestations; Most commonly pneumonia in the form of exudation of the lung with hyaline membrane formation rendering most of the lung non-functional causing hypoxemia and anosmia, all these findings that affect the lungs’ functions and lowers blood-oxygen saturation levels play a huge role in defining prognosis of the disease.
Keywords: COVID-19; Structure; Pneumonia; Hypoxemia; CO-RADs; Anosmia
Abbreviations
GGO: Ground Glass Opacity; RT-PCR: Reverse Transcription Polymerase Chain Reaction; COVID-19: Coronavirus Disease Of 2019; OFR: Open Reading Frame; ACE2: Angiotensin Converting Enzyme 2; TMPRSS2: Transmembrane Protease, Serine 2; ARDS: Acute Respiratory Distress Syndrome
Introduction
Corona virus family has caused three massive outbreaks over the past two decades, SARS (Severe Acute Respiratory Syndrome) in 2002, MERS (Middle East Respiratory Syndrome) in 2012 and now COVID-19 (Corona Virus Infection Disease 2019) which is caused by SARS-COV-2 [1,2]. Its genome consists of 14 ORFs (Open Reading Frames), most of them encode (nsp1-16) playing an important role in viral replication [2,3]. The rest encode accessory proteins and 4 structural proteins (spikes, envelope, membrane and nucleocapsid) [4]. Corona affects mainly the respiratory system and oxygen level in blood causing hypoxemia. Corona has a high mortality rate all over the world, especially in old age. The respiratory system’s structural and functional units are the alveoli. The respiratory portion of the respiratory system starts with the respiratory bronchiole and then moves on to the alveolar ducts, alveolar sacs, and eventually the alveoli, where there is a large exchange of gases. Patients with COVID-19 suffer from cough, lung ground-glass opacities and symptoms of pneumonia. This indicates direct transmission of SARS-COV-2 via respiratory droplets [5,6].
Background on Normal Lung Structure
The lungs are the main organs affected by COVID-19, Each lung lobe is aerated by a secondary bronchus, lobes are further divided into bronchopulmonary segments; smaller and pyramidal in shape, and every lung has ten bronchopulmonary segments, each aerated by a tertiary bronchus [7]. The nose, nasopharynx, larynx, trachea, and a series of successively narrowing segments of bronchi and bronchioles make up the conducting portion of the respiratory system. The terminal bronchiole is where the conducting portion of the bronchiole ends. The respiratory system begins with the respiratory bronchiole and progresses to the alveolar ducts, alveolar sacs, and finally the alveoli, where a significant amount of gas exchange takes place [8]. Alveolar ducts are created when respiratory bronchioles divide distally. Alveolar ducts do not have their own walls but are formed by multiple alveolar openings. The alveolar sac is made up of these clusters of alveoli. The respiratory system’s structural and functional units are the alveoli. An adult human has about 300 million alveoli, which equates to around 80 square meters of surface area for gaseous exchange. Each alveolus is lined by two types of epithelial cells. They are type I pneumocytes (alveolar lining cells) and type II pneumocytes [9].
Mode of Infection and Pathogenesis of COVID-19
Symptoms of COVID-19 include cough, anosmia, ageusia, shortness of breath and acute respiratory distress or even complete respiratory failure (in severe cases). This indicates direct transmission of SARS-COV-2 via respiratory droplets which cause human-to-human spread of infection [1,3,10-12]. There are other routes of transmission were reported during the pandemic such as aerosol, direct contact with contaminated surfaces, and fecal–oral transmission [5,6].The contaminated droplets in air reach all parts of the bronchial tree and mainly situated in the lower lobes of the right and left lungs. After the viral entry the spike interacts with ACE2 and TMPRSS2 on cell membranes of the human cells to enter and releases its genome into cytosol [13-15]. Shortly after that, ORF1a and ORF1b (open reading frame 1) are translated into replicate proteins that form RNA-dependent RNA polymerase which facilitates viral replication of genomic and sub-genomic RNA that lead to releasing more and more generations of the virus [4,16,17]. The process of replication stimulates the immune response and releasing of pro-inflammatory mediators which cause lung pathology through stimulation of endothelial cell damage, vasodilatation, and Inflammatory cells, mainly neutrophils and macrophages [18-23]. Those steps cause Vasodilatation with vascular leakage leading to edema and deficient gas exchange leading to hypoxia, leading to respiratory/organ failure. This explains the reason why patients with COVID-19 suffer from high fever, lymphocytopenia, neutrophilia and elevated inflammationrelated indices [10,23-28]. Generally, SARS-CoV-2 infection may vary from asymptomatic cases to severe pneumonia that may increase mortality [14]. Cough, fever, and shortness of breath, sore throat, hemoptysis, and anosmia have a different distribution in mild and severe cases [29,30]. Mild symptoms can be reported in most of the patients (about 80%) especially who are young adult or children [31], imaging shows no signs of pneumonia [32] and lab diagnosis shows a normal level of d-dimmer less than 0.5μg/ml [33,34]. Moderate cases develop down lung inflammation and alveolar-capillary destruction [35] the latter might be named pneumolysis, leading to progressive hypoxemia and pulmonary shunt [36]. Severe and critical cases are associated with older age, co-morbidities (such as cardiovascular disease, hypertension, obesity, and chronic lung disease [37], several lung CT features as bilateral ground-glass opacity, or consolidation shadow [32]elevated d-dimmer (more than 0.5μg/ml) which may lead to an imbalance of coagulation and fibrinolysis in the alveoli and if its level is greater than 1μg/ml is a sign of poor prognosis for patients [34], leukopenia and severe lymphopenia [24]. So critical cases may develop complications as Acute Respiratory Distress Syndrome (ARDS) needing prolonged ventilator support, cardiac rhythm disorders which may lead to shock and finally death [38]. For all of these hospitalization rate increases and becomes important in critical cases [23].
Structural Lung Changes in CT Scans
According to the WHO, the golden diagnostic standard in Covid-19 suspected patients is CT scans [39], as it shows 80-90% accuracy and 82.9-96% specificity [40] (RT-PCR) shows only 37- 83% sensitivity [41] Reporting of Covid-19 cases will play a huge role in controlling the pandemic epidemiologically [42] an initiative to a standardized way of reporting COVID-19 confirmed cases is COVID-19 Reporting And Data System (CO-RADs) which grades depending on how likely there is a Covid-19 infection, it uses clear terms and high accuracy in predicting moderate to severe infection [43-45]. CO-RADs have seven categories classified from no evidence of COVID-19 infection (CO-RADs 1) to confirmed Covid-19 infection (CO-RADs 6) with categorization start point at 0 grade which indicates a healthy lung [46].
CO-RADs 0: This value is given in case none of the other categories can be allocated for the scan or the scan is not clear or interrupted, some parts of the lung might be missing in the scan, scans in this category must not be taken as the final scan and it should lead to a second scan [46].
CO-RADs 1: This category includes scans that are less likely to indicate a COVID-19 infection, normal scans or unequivocally noninfectious diseases; emphysema, lung tumors or fibrosis; early scans have to be put into consideration when COVID-19 is still mild with no clinical symptoms (Figure 1) [45].
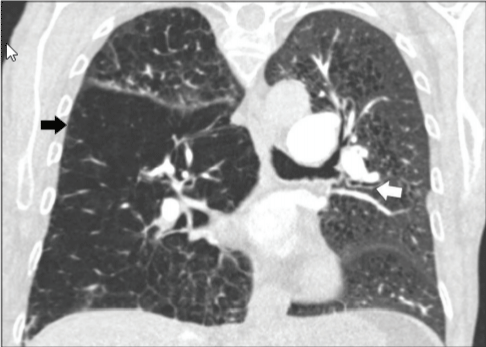
Figure 1: CO-RADs 1, the lung is normal, or it has non-infectious conditions
as (emphysema, fibrosis, lung tumors, Sarcoidosis or heart failure), this CT is
for 58 years old emphysema patient with bronchial thickening [46].
CO-RAD 2: Includes infectious diseases of the lung showing (ground-glass opacities, bronchitis, bronchopulmonary pneumonia, lobar pneumonia and pulmonary abscess) but not COVID-19, this category has low level of COVID-19 infection (Figure 2 and 3) [47].
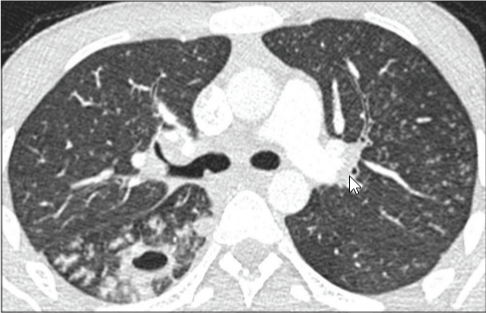
Figure 2: CO-RADs 2, lung with tree in bud sign, the patient is 27 years with
symptoms of cough and fever, he had active tuberculosis [46].
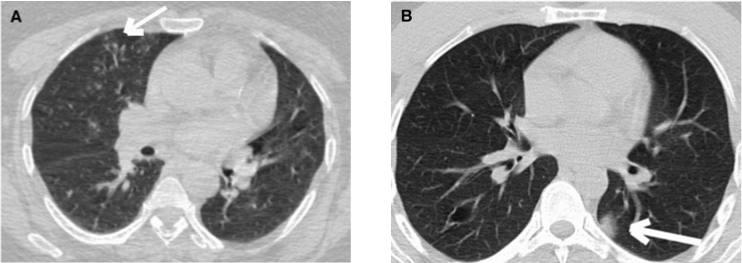
Figure 3: CO-RADs 2; a). Is for a 52-year-old female with confirmed Covid-19
infection with tree in bud. b). Is for a 41 years old male with confirmed
Covid-19 with unifocal ground-glass appearance [47].
CO-RADs 3: Includes findings indicative of a COVID-19 infection but can also indicate other infectious and non-infectious diseases (peri-hilar ground glass, homogenous and extensive ground glass appearance, ground glass opacities with interlobular interstitial thickening and patterns of organizing pneumonia with no characteristic findings of COVID-19 as ground-glass opacity near the pleura), this category implies ambiguous findings of COVID-19 infection (Figure 4) [46].
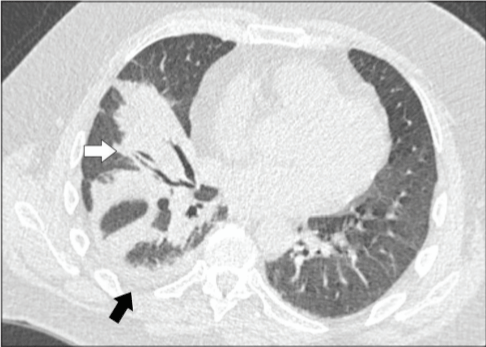
Figure 4: CO-RADs 3; Unilateral peri-broncho-vascular consolidation with
no ground glass appearance, scan for 52 years old patient with one typical
finding of COVID-19, later he had one positive and two negative swabs for
COVID-19 [46].
CO-RADs 4: includes findings found in COVID-19 infection and at the same time overlap with other viral pneumonias; findings are atypically distributed, no contact with visceral pleura with strict unilateral involvement, with main distribution peri-bronchovascular or superimposed on severe diffuse pre-existing pulmonary abnormalities (Figure 5) [46], this category indicates high level involvement of COVID-19.
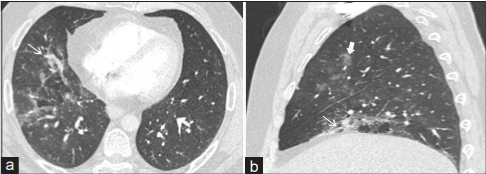
Figure 5: CO-RADs 4: CT scan (a and b) presents unilateral
peribronchovascular consolidation and unilateral ground glass opacity; scans
are for 52 years old male who had symptoms of Covid-19 for two weeks and
was later confirmed by PCR [46].
CO-RADs 5: Features included in this category are divided into two groups: Mandatory features that must be present in all scans and three confirmatory features, present of one confirmatory feature is necessary for grading: Mandatory features include (Ground-glass opacities with or without consolidation near visceral pleura with multifocal bilateral distribution and sub-pleural sparing may be present or absent) and Confirmatory features: 1. Multiple groundglass opacities, circular or half-circular with weak limitation of the borders it may also appear as complete circles outlining edges of adjacent pulmonary lobules. 2. Crazy paving pattern; formed of interlobular interstitial thickening and ground glass opacities, and 3. Organizing pneumonia which shows reversed halo sign, groundglass consolidation that is associated with sub-pleural consolidation and air bronchogram. In addition to bands of ground-glass, it may be with or without consolidation, and arching pattern with subpleural contact. (Thickened vessels may occur in any of the three confirmatory patterns) this category includes scans with the highest level of suspicion of COVID-19 (Figure 6) [45].
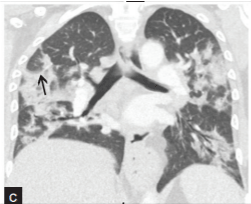
Figure 6: CO-RADs 5: CT scan shows bilateral ground-glass appearance
with consolidation, the scan belongs to a 52-year-old female admitted with
organizing pneumonia [46].
CO-RADs 6 (Figure 7): This category includes scans of patients who were confirmed to be infected by COVID-19. The infection was confirmed using Reverse Transcription-Polymerase Chain Reaction (RT-PCR) technique. N.B. Ground glass opacity is characteristic of CO-RADs 3, but if it has centrilobular involvement then it is CORADs 2, if it is located near the visceral pleura or fissures then it is CO-RADs 4, if it is bilateral and multifocal then it is classified as CO-RADs 5 (ground-glass opacity must be present with or without consolidation and located close to the pleural (ground-glass opacity must be present with or without consolidation and located near the pleural surface [45,46]. Wuhan University classifies Covid-19 disease into five distinct stages: Ultra-early stage, early stage, rapid progression stage, consolidation stage and dissipation stage [44]. CO-RADs classification, on the other hand, classifies the disease into only three stages: early stage, intermediate stage, and final stage. These stages feature ground-glass opacity, lines forming crazy-paving pattern, and features resembling organizing pneumonia respectively [45,48].

Figure 7: CO-RADs 6: there is severe GGO with consolidation and positive
PCR [46].
Post-Partum Lung Structural Changes
The affected combined lung weighed (1000-3110 g) [49]. The affected lungs were moderately adherent to their pleura, patchy and hard inconsistency. They also showed grayish whitish lesions and dark red bleeding. In addition to that, the affected lungs had exudative and proliferative diffuse alveolar damage. When sliced, fiber bundles were observed. The results of the autopsy have reflected the distribution of imaging changes and showed the further development of the lesions. If we are to consider the correspondence between the ground-glass opacities, which were found in scans and the graywhite alveolar lesions found after the autopsy, we can conclude that COVID-19causes inflammatory reactions that are characterized by deep respiratory tract and alveolar damage. There is not much pleural effusion, suggesting that there is no serious inflammation in lung disease. Pericardial effusion, moderate epidural edema, and myocardial red flesh [50]. No macroscopic thrombo-emboli or areas of infarction were seen [51]. These findings suggested viral pneumonia. On examining specimens of affected lungs under the microscope, A) there is edema of parenchymal tissue with thickening, the parenchymal tissue was destroyed due to extensive necrosis. B) It was also found that the alveolar structure was either damaged or collapsed; some patent alveoli were filled with mucoid substance; the exudate of the congested capillaries. C) The alveolar lining showed alveolar epithelial hyperplasia. D) Deep pulmonary vessels showed fibrinoid necrosis. E) The fibrous scaffolding of the lung was lost. F) Chronic inflammatory cells were seen in the exudate and the virus has been detected in huge numbers using the electron microscopy techniques [52].
Mortality Based on the Findings
CT scans and radiographs are excellent prognosis predictors and give hints on the level of severity of the disease [53], CT scans mainly diagnose moderate to severe cases while Radiographs may be asked to diagnose mild cases [45], thus they are the main tools used in assessing the course of the disease in most studies.Hu et al. also describe in their experiment that from 73 patients that had their CTs examined after their death, 58 of them had a single CT scan before their death, 11 had two CT scans, three had three CT scans and only one had four CT scans, lung involvement was measured by the involvement of the 5 lung lobe; 0-no involvement (0%)/1-minimal involvement(1-25%)/2-mild involvement (26-50%)/3-moderate involvement (51-75%)/4-severe involvement (76-100%) [54], this score was summed with a score that describes the severity of lung involvement; 0-none/(1-5) minimal/(6-10) mild/(11-15) moderate/ (16-20) severe-white lung [55].the major findings in the patients who died from Covid-19 were Ground-glass opacity with consolidation and ground-glass opacity which is caused by the structural change in blood vessels and formation of exudate in the alveolar spaces with hyaline membrane formation [56,57]. Patients who had a second CT scan had increased ground glass with consolidation in all of the 5 lobes (43-57%), GGO in all of the right lobes and the left upper lobe (16-25%), consolidation in the lower left lobe (19%) with a mean five days interval between the scans (the total involvement score was 12.97±5.87, 11% of the patients was rated as severe in the initial CT scan while 66% of the patients was rated severe on the follow up [55], these data shows that increase in the severity of the lung happens in a short span of time, Also higher involvement with higher severity degree in a short span of time indicates poor prognosis and high mortality chance.
Conclusion
COVID-19 caused a pandemic that froze our daily normal life by isolating ourselves in homes and hospitals, this is due to the high mortality rate of the disease, a mortality rate that is attributed to many internal and external factors, internal factors are summarized as (each individual immune response, tolerance to hypoxemia and overall structural changes to the lung made by the virus), the virus is capable of infecting many organs of the body but most importantly the lung causing distinct features that enable us to differentiate it from so many more types of infectious and non-infectious diseases, typical features are 1.Ground-Glass Opacity (GGO) it could be unilateral unifocal, unilateral multifocal or multilateral multifocal with the latter being the most serious and indicative of the disease [58]. 2. Consolidation and air bronchogram which happens as a result of filling the alveolar spaces with exudate, blood or neoplastic cells, the consolidation in COVID-19 is mainly peripheral or subpleural. 3. Crazy paving signs, which represent alveolar and interstitial edema [59]. 4. Nodules which are opacities in the CT scan less than 3cm [60]. 5. Subpleural curvilinear lines are demonstrated as thin lines (1- 3mm) that are parallel to and 1cm away from the visceral pleura [61]. All of these signs are used to identify and assess COVID-19 infection and put into perspective a prognosis to the course of the disease.
References
- Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003; 361: 1319-1325.
- Zhang Y-Z, Holmes EC. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell. 2020; 181: 223-227.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020; 95: 565-574.
- Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nature Reviews Microbiology. 2009; 7: 439-450.
- Li Y, Huang X, Yu IT, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005; 15: 83-95.
- Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016; 92: 235-250.
- Chaudhry R, Bordoni B. Anatomy, Thorax, Lungs. StatPearls. Treasure Island (FL). 2021.
- Patwa A, Shah A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J Anaesth. 2015; 59: 533-541.
- Haddad M, Sharma S. Physiology, Lung. StatPearls. Treasure Island (FL). 2021.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020; 323:1061-1069.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020; 382:1199-1207.
- Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020; 395: 514-523.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581: 215-220.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 588: E6.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367:1260-1263.
- Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006; 80: 5927-5940.
- Wu H-Y, Brian DA. Subgenomic messenger RNA amplification in coronaviruses. Proceedings of the National Academy of Sciences. 2010; 107: 12257-12262.
- Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003; 200: 282-289.
- Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003; 361: 1773-1778.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017; 39: 529-539.
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020; 368: 473-474.
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46: 846-848.
- Yang X, Yu Y, Xu J, Shu H, Xia JA, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020; 8: 475-481.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020; 395: 507-513.
- Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020; 80: e14-e18.
- Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020; 20:1043-1050.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180: 934-943.
- Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020; 15: e0235458.
- He X, Cheng X, Feng X, Wan H, Chen S, Xiong M. Clinical Symptom Differences between Mild and Severe COVID-19 Patients in China: A Meta- Analysis. Frontiers in Public Health. 2021; 8.
- Harahwa TA, Lai Yau TH, Lim-Cooke MS, Al-Haddi S, Zeinah M, Harky A. The optimal diagnostic methods for COVID-19. Diagnosis (Berl). 2020; 7: 349-356.
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020; 94: 44-48.
- Zhang J, Wang M, Zhao M, Guo S, Xu Y, Ye J, et al. The Clinical Characteristics and Prognosis Factors of Mild-Moderate Patients with COVID-19 in a Mobile Cabin Hospital: A Retrospective, Single-Center Study. Frontiers in Public Health. 2020; 8: 264.
- Favresse J, Lippi G, Roy PM, Chatelain B, Jacqmin H, Ten Cate H, et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018; 55: 548-577.
- Yu HH, Qin C, Chen M, Wang W, Tian DS. D-dimer level is associated with the severity of COVID-19. Thromb Res. 2020; 195: 219-225.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020; 383:120-128.
- Zubieta-Calleja G, Zubieta-Deurioste N. Pneumolysis and “Silent Hypoxemia” in COVID-19. Indian Journal of Clinical Biochemistry. 2021; 36:112-116.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA. 2020; 323:1239-1242.
- World Health O. Laboratory testing for coronavirus disease ( COVID-19) in suspected human cases: interim guidance, 19 March 2020. Geneva: World Health Organization. 2020.
- Majidi H, Niksolat F. Chest CT in patients suspected of COVID-19 infection: A reliable alternative for RT-PCR. Am J Emerg Med. 2020; 38: 2730-2732.
- Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020; 94: 107-109.
- Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology: Cardiothoracic Imaging. 2020; 2: e200152.
- Inui S, Kurokawa R, Nakai Y, Watanabe Y, Kurokawa M, Sakurai K, et al. Comparison of Chest CT Grading Systems in Coronavirus Disease 2019 (COVID-19) Pneumonia. Radiol Cardiothorac Imaging. 2020; 2: e200492.
- Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019- nCoV) infected pneumonia (standard version). Mil Med Res. 2020; 7: 4.
- Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology. 2020; 296: E97-e104.
- Penha D, Pinto EG, Matos F, Hochhegger B, Monaghan C, Taborda-Barata L, et al. CO-RADS: Coronavirus Classification Review. J Clin Imaging Sci. 2021; 11: 9.
- Özel M, Aslan A, Araç S. Use of the COVID-19 Reporting and Data System (CO-RADS) classification and chest computed tomography involvement score (CT-IS) in COVID-19 pneumonia. La radiologia medica. 2021; 126:679- 687
- Bertoncelli D, Guidarini M, Della Greca A, Ratti C, Falcinella F, Iovane B, et al. COVID19: potential cardiovascular issues in pediatric patients. Acta Biomed. 2020; 91: 177-183.
- Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A, et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology. 2021; 88: 56-68.
- Chen W, Pan JY. Anatomical and Pathological Observation and Analysis of SARS and COVID-19: Microthrombosis Is the Main Cause of Death. Biological Procedures Online. 2021; 23: 4.
- Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. Journal of Clinical Pathology. 2020; 73: 840-844.
- Wu J, Yu J, Zhou S, Zhang J, Xu J, Niu C, et al. What can we learn from a COVID-19 lung biopsy? International Journal of Infectious Diseases. 2020; 99: 410-413.
- Yasin R, Gouda W. Chest X-ray findings monitoring COVID-19 disease course and severity. Egyptian Journal of Radiology and Nuclear Medicine. 2020; 51: 193.
- Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020; 295: 200463.
- Hu Y, Zhan C, Chen C, Ai T, Xia L. Chest CT findings related to mortality of patients with COVID-19: A retrospective case-series study. PLoS One. 2020; 15: e0237302.
- Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020; 36: 21-23.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8: 420-422.
- Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. Journal of the American College of Radiology. 2020; 17: 701-709.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology. 2008; 246: 697-722.
- Franquet T. Imaging of Pulmonary Viral Pneumonia. Radiology. 2011; 260:18-39.
- Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features. Investigative Radiology. 2020; 55: 257-261.