Short Communication
Clin Oncol Res. 2018; 1(2): 1007.
Study on shRNA Mediates PD-1 Gene Silencing Enhances Anti-Lymphoma Efficacy of CAR-T Cells
Wang J, Wang H, Yan Z, Wang Y*, Yu L and Zhu J
Institute of Biomedical Engineering and Technology, Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, China
*Corresponding author: Wang Y, Institute of Biomedical Engineering and Technology, Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, China
Received: October 30, 2018; Accepted: December 04, 2018; Published: December 11, 2018
Short Communication
CAR-T therapy has made remarkable achievements in the treatment of lymphoma, but there are still some problems to be resolved. In clinical trials, a sample of lymphoma patients will be found to express a large amount of PD-L1 in the tumor cells of the patient, resulting in inadequate anti-tumor effect or tumor recurrence after treatment. This PD-1/PD-L1 pathway mediates tumor cell immune evasion and reduces therapeutic effects. In response to this problem, we constructed a CAR-T cell that silences the expression of PD-1 gene using small interfering RNA technology. When the killing effect of CAR-T cells and tumor cells occurs, the expression of PD-1 is reduced and minimized. The anti-tumor effect of CAR-T cells is reinforced by the effects of immune escape [1,2].
First, we performed a medium pumping of plasmids: PLV-CD19 CAR, PLV-ShPD1/CD19 CAR, PLV-CD19, and PLV-CD19-PD-L1 to obtain sufficient amount of subsequent viral packaging. The concentration values are determined by a concentration analyzer listed in Table 1. The lentivirus was packaged by a three-plasmid packaging system, and the virus supernatant was subsequently concentrated and purified. The titer of the concentrated virus was shown in Table 2. Then we obtained T cells by separating and sorting donor peripheral blood, and transduced with CAR lentivirus. The CAR-T cells prepared after transduction were detected by flow cytometry for transduction efficiency cell typing. The results confirmed that the prepared CAR-T cells have high transduction efficiency, and the CD4+ and CD8+ cell ratios are similar, which can be characterized in vitro and in vivo. The shRNA-mediated PD-1 gene silencing efficiency, cell killing and cytokine release was also examined. Finally, the T cell proliferation function was evaluated.
Plamides
Concentration
(mL-1)
PLV-CD19 CAR
2078
PLV-ShPD1/CD19 CAR
1987
PLV-CD19
2651
PLV-CD19-PD-L1
2283
Table 1: Concentration of plamides.
Virus
Titer
109mL-1
PLV-CD19 CAR
4.12
PLV-ShPD1/CD19 CAR
3.87
PLV-CD19
2.73
PLV-CD19-PD-L1
3.11
Table 2: Titer of the concentrated virus.
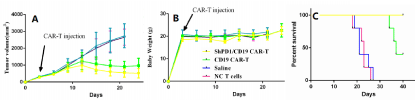
The K562-CD19-PD-L1 double antigen target cells were expanded in 1640 medium, then centrifuged at 1200rpm, washed three times with PBS solution, counted under a microscope, and resuspended in saline to a cell concentration of 5x107cells/mL. 500μL of the cell suspension was aspirated with a syringe and inoculated into the skin of 5 NOD-SCID mice in equal portions to observe the tumor formation. When the tumor volume of the mouse reached 300mm-3, the tumor tissue block was taken out, the necrotic tissue was removed, and the tumor block was placed in a sterile petri dish containing a little physiological saline. Tumor tissue with good growth, no necrosis and reddish color resembling fish flesh was selected from the culture dish, and cut into small pieces of about 30mm3 using small scissors, carefully picked up with tweezers and sent to the puncture needle. The tumor block was injected into the skin of the mouse with a puncture needle to complete the inoculation of the tumor mass. The growth state of the mice and the size of the tumor were observed every two days. When the tumor volume reached 200-300 mm3, the mice were divided into 4 groups with an injection machine, 4 in each group. The experimental group was: ShPD1/CD19 CAR-T cells, control group: CD19 CAR-T cells, negative control group: NC-T cells, blank group: normal saline. Each of the 4 groups of mice was injected with 100μL of the corresponding suspension in each tail vein. Continuous observation of mouse survival status. The experimental results showed that the prepared ShPD1/CD19 CAR-T can effectively reduce the PD-1 expression of CAR-T cells with a silencing efficiency of 67.3%. From the cell killing and factor experiments, it can be seen that the killing effect of CD19CAR-T is reduced due to the expression of PD-L1 on the target cells, and the PD-1 shRNA original is added to reduce the expression of PD-1 in CAR-T cells. Effective and timely killing in the face of target cells expressing PD-L1. At the same time, due to the decrease of PD-1 expression, the cell proliferation ability is enhanced, and the anti-tumor ability of the effector cells is enhanced from the side. Finally, we used the method of PD-L1 and CD-19 overexpressing K562 cells to form a tumor model. After successful model establishment, CAR-T cells were infused to evaluate tumor anti-tumor effects by monitoring tumor volume and survival curve. From the results, we can easily conclude that the expression of PD-1 in CD19 CAR- T cells decreased due to the introduction of PD-1 shRNA. It reduces immune evasion, enhances its ability to reproduce in the body, and achieves a sustained anti-tumor effect. The experimental results were presented in Figure 1. It can be observed in Figure 1A that, compared with the control group, after injecting CD19 CAR-T cells for ten days, the tumor mass not only does not grow up, but shrinks. However, the tumor mass has significantly reduced for the group injected with ShPD1/CD19 CAR-T cells. Figure 1B shows that there are no significant change in body weight of the mice in each group, indicating that the toxicity of both CAR-T cells is relatively low. Figure 1C reflects that the control mice survived for 19-28 days, the group infused with the CD19 CAR-T cell survived for 35-40 days, and the group injected with ShPD1/CD19 CAR-T cells survived for more than 40 days.