Abstract
Background: Iodine deficiency is one of the most neglected nutritional deficiencies. It may result in development of goitre and other Iodine Deficiency Disorders (IDD). The goitre prevalence reflects the iodine deficiency in past while Urinary Iodine Excretion Levels (UIEL) gives the current status of iodine nutrition.
Objective: To determine the status of biochemical iodine deficiency in school children and to find out the demographic and dietary factors associated with iodine deficiency disorders.
Method: This study was conducted among school children of 1st to 5th standard (6–12 yrs.) from 1st January 2013 to 31st December 2013. A total of 907 students of seven schools were included using Probability Proportional to Size (PPS) sampling method. According to recommendations, 10% of urine samples from total children interviewed i.e. 90 were analyzed. Urine samples were tested for estimation of UIEL. Statistical analysis was done using SPSS version 20.
Results: Age distribution of the study population shows that majority of the students (30.5%) belonged to 11-12 years, and large number of students (56.2%) was males. Median UIEL for all children was found to be 140 µg/l. The proportion of children having normal range of >100 µg /l were 76.7%. Children with mild, moderate and severe grades of UIEL were 7.8%, 5.5% and 10.0% respectively. Prevalence of iodine deficiency, calculated by proportion of children having UIEL of <100 µg/l, was 23.3%.
Conclusions: Median UIEL was found to be 140 µg/l, which was higher than the level accepted for the definition of iodine deficiency, i.e. a concentration of less than 100 µg/l. Hence, the area would be categorized as having “No biochemical iodine deficiency”.
Keywords: Iodine Deficiency Disorders; Urinary Iodine Excretion Level; Biochemical Iodine Deficiency
Introduction
Iodine deficiency is one of the most neglected and wide spread of all nutritional deficiencies, posing a hindrance to human developers.
Iodine is required for the synthesis of thyroxine (T4) and triiodothyronine (T3). These hormones are very important in the regulation of proteins, carbohydrates and fats metabolism that affect almost all the activities in the body.
As far as the magnitude of the problem is concerned, the countries of South East Asia present a particularly urgent challenge for the control of Iodine Deficiency Disorders (IDD). Many countries in South East Asia have IDD as a significant health problem. According to World Health Organization (WHO), iodine deficiency occurs in 130 countries around the world, and 2.2 billion people (38% of the world’s population) live in iodine deficient areas [1].
In India, IDD has been identified as a public health problem. The world’s most intense goitre belt is in India stretches 2400 Kms from Kashmir in the North West to the Naga Hills in the East. In addition to the known Himalayan endemic belt, iodine deficiency and endemic goitre has been reported from many other states in the country as well. Time and again new pockets of iodine deficiency are being identified. Surveys conducted in India have revealed that out of the 325 districts surveyed in India, 263 districts are IDD-endemic, i.e. the prevalence of IDD is above 10 per cent in the population [2]. Out of total population of India (approx 1200 million) more than 200 million are at risk of IDD [3]. WHO recommends that for assessment of Iodine deficiency in an area, children in the age group 6-12 years should be surveyed [4].
The other methods of iodine supplementation are injection of iodized oil, addition of iodine to bread and iodination of irrigation water but these methods are not universally applicable [5].
The goitre prevalence reflects the iodine deficiency in past while UIEL gives the current states of iodine nutrition and both cannot be compared at a time.
A number of survey and research activities have been carried out in various parts of the world and in our country aimed to assess the magnitude of the problem and status of the NIDDCP. However, data are deficient on various aspects of the problem; therefore, there is a need for further research in a number of fields related to IDD, so that this data can be made available to planners and policy makers. Paucity of clinical, laboratory and epidemiological data in Aligarh makes it difficult to understand the magnitude of problem. The present study is an attempt to carry out an in depth assessment of biochemical iodine deficiency in school children.
Material and Methods
The present study was conducted among school children (6–12 yrs). School children were chosen for the study because they are highly vulnerable to IDD, representative of the community and easily accessible. Three government and four private schools in Aligarh were selected.
Sampling unit
1st to 5th standard children of the schools (age group 6-12 years) were the “sampling units” for study conducted in schools. This is the preferred group as it is usually accessible. There is a practical reason for not measuring very young age groups. The smaller the child, the smaller the thyroid and it is more difficult to perform palpation [4]. In the selected schools, almost every child of 1st standard had completed six years of age and most of the children of 5th standard were completing twelve years of age.
Study duration
This study is a part of a longitudinal study entitled “A study of iodine deficiency disorders and the impact of intervention package to improve consumption of iodized salt”. The data was collected in schools over a period of one year from 1st January 2013 to 31st December 2013. Different schools were approached over this period of time as per the convenience of the investigator and school authorities. Taking into account the limited resources, this method was adopted.
Sample size
Sample Size: Directorate General of Health Services found a goitre prevalence rate of 12% in Aligarh District [6]. This prevalence of goitre was used for calculating the minimum sample size in our study. Taking the value of prevalence ‘p’ as 12% and relative error (l) 20 % of ‘p’, the sample size (N) was calculated as: [7]
N = 4 × p × q / l2
Where p (%) = prevalence, q (%) = 100-p,
l = relative error
N = 4 × 12 × 88/ (0.2 × 12)2
N = 733
Taking into consideration 20% non response / non co-operation rate, the above sample size was increased by 20% then the total sample size was:
N = 733 + (0.2 x 733)
N = 879
However, a total of 950 subjects were included in the study.
Plan of study
Schools were contacted several days before the study began to inform the principals of the schools, the study purpose and to get consent from them as well as parents/guardians of children. In consultation with principal, a suitable date (a day on which the attendance in the school was maximum, preferably early in the week, avoiding national and state holidays), time and place for interviewing and examining the children were chosen. As a part of ethical considerations, they were briefed about presentation of IDD, and its consequences and methods available for its prevention especially health benefits of taking iodized salt in diet, food items which prevent the utilization of iodine in the body. This helped us having their maximum participation for conducting the study in school children and it also ensured good attendance of students.
The school authorities were asked to provide us the list of students who were enrolled in classes from 1st to 5th standards and were in the age group 6-12 years. This list was the sampling frame of our study. We requested for school records showing their dates of births. The age was classified according to their dates of births.
Sampling procedure
The required sample was selected by “Multistage sampling” by doing a sub sampling. In the first stage, schools were selected over a period of time, after permission from school authorities. In the second stage, a list of students in class 1st to 5th standard in age of 6-12 years was obtained. Our “sampling frame” consisted of number of students selected from one school. As per Probability Proportional to Size (PPS) method, number of students in a school was proportional to the strength of total number of students (6-12 years) from all schools. The next stage was to select students in a school. With the help of random number table, a random sampling method was applied to select the final numbers of students from a school to be included in the study. We assigned a serial number to each student in that school.
It is recommended that from a minimum of 10% of the children being surveyed, urine samples should be collected to get a valid estimate of iodine status in a community [8]. Keeping in view the above recommendations, we took 10% of urine samples from total children interviewed i.e. 90 from 907. We increased it further to 93 to cover for dropouts and in order to ensure that 90 samples would be transported safely and tested.
The numbers of students, required for urine collection in a school were proportional to size of student’s sample selected for goiter examination in that particular school (Table 1). We already had a list of these students from each school and we assigned them a fixed serial number at random, starting from any student. The required numbers of children for urine examination from a school were picked up by using the systematic random sampling method.
School No.
Total children
enrolled in schools
Children studied
Urine samples
collected
1
215
124
13
2
38
22
3
3
313
180
18
4
598
344
35
5
87
50
5
6
290
167
17
7
35
20
2
Total
1576
907
93
Table 1: Selection of students for urine collection in Aligarh.
Urine collection from the study subjects was performed only after getting permission from principals and their guardians. Sterile plastic urine containers were labeled and distributed to the students. Instructions to the students were given regarding the collection of urine samples. Five milliliters of fresh “on the spot” urine sample was collected from each selected subject and labeled with unique identification number corresponding to the student’s name. Few drops of toluene were added to each urine sample to inhibit bacterial growth and to minimize bad odour.
Urine samples were kept in refrigerator and analysed in the laboratory of the Department for estimation of Urinary Iodine Excretion Levels (UIEL) using the Wet Digestion Method of the Sandell-Kolthoff reaction, in which iodine was determined from its catalytic reduction of ceric ammonium sulfate in the presence of arsenic acid [9]. The results were expressed as microgram of iodine per litre of urine (µg/l).
A pooled urine sample was prepared for Internal Quality Control (IQC) assessment. The IQC sample was analysed 25 times with standards and blank with duplicate. This IQC sample having a known concentration range of iodine content was run with every batch of test samples. If the result of IQC sample was within range (i.e. Mean ± 2 SD), the test was deemed in control and if the results were outside the range (The reason being contamination of urine sample by water or any error in test procedure), the whole batch was repeated. A graph was plotted. On Y axis we had Optical Density (OD) at 420 nm and on X axis we had concentration of iodine in urine in microgram/dl. Final results of UIEL were given in µg/l by multiplying with a factor of 10 to µg/dl.
Out of 93 samples collected for testing, one was excluded due to the volume of the urine sample being inadequate, another sample could not be analyzed due to sediments and turbidity and a third sample was wasted due to the breakage of the glass test tube during the test. Therefore, finally 90 samples were tested for urinary iodine estimation.
Every child was asked to bring approximately two teaspoons (10 gm) of salt being used for cooking in their respective households, in labeled auto sealed polythene pouches. For community or population surveys, 10 gm salt samples are sufficient [10].
The study was approved by Institutional Ethics Committee.
Inclusion criteria
- Students of schools whose principals gave consent to our study.
- Students of classes from 1st to 5th standard who were of age group 6-12 years.
- Students whose parents/guardians gave consent to our study.
Exclusion criteria
- Students not attending the school on the day of study.
- Students whose parents/guardians did not give consent to our study.
- Students aged less than 6 years and more than 12 years.
Statistical analysis was done using SPSS version 20. Chi-square was used to find out association and p value < 0.05 was considered as significant.
Results
The Urinary Iodine Excretion levels (UIEL) of 90 urine samples in schools is shown in (Table 3). The analysis of distribution of UIEL revealed values lower than 100 µg/l in 23.3% of samples; 15.5% of them being lower than 50 µg/l. Iodine deficiency is defined by the WHO as Median Urinary Iodine Concentration (MUIC) that falls below 100 µg/l, while a MUIC of 50–99 µg/l, 20-49 µg/l, and <20 µg/l indicates mild, moderate, and severe iodine deficiency, respectively [8].
Median Urinary Iodine Concentration (µg/L)
Iodine Intake
Iodine Status
= 300
Excessive
Risk of adverse health consequences (iodine-induced hyperthyroidism, autoimmune thyroid disease)
200-299
Above Requirements
May pose a slight risk of more than adequate iodine intake in these populations
100-199
Adequate
Adequate iodine nutrition
50-99.9
Insufficient
Mild iodine deficiency
20-49.9
Insufficient
Moderate iodine deficiency
< 20
Insufficient
Severe iodine deficiency
Table 2: Median urinary iodine concentration and iodine status.
S. No.
Schools
Urinary Iodine Excretion Level (µg/l)
Total
(%)
<20
(%)
20-49.9
(%)
50-99.9 (%)
= 100
(%)
Green Fields Public School
2
(22.2)
1 (20.0)
2 (28.6)
8
(11.6)
13
(14.4)
Madinatul Uloom College
0
(0.0)
0
(0.0)
0
(0.0)
2
(2.9)
2
(2.2)
Krishna International School
1
(11.1)
2 (40.0)
0
(0.0)
15
(21.7)
18
(20.0)
Iqra Public School
2
(22.2)
1 (20.0)
2 (28.6)
30
(43.5)
35
(38.9)
Primary School, Nagla Qila
1
(11.1)
0
(0.0)
1 (14.3)
3 (4.30
5 (5.5)
Union School for Girls, A.M.U.
2
(22.2)
0
(0.0)
2 (28.6)
11
(15.9)
15
(16.7)
Primary School Gorai
1
(11.1)
1 (20.0)
0
(0.0)
0
(0.0)
2
(2.2)
Total
9
(10.0)
5
(5.5)
7
(7.8)
69
(76.7)
90
(100.0)
Table 3: Distribution of urinary iodine excretion levels in schools.
In the present study, Median Urinary Iodine Level (MUIEL) for all children was found to be 140 µg/l, which was higher than the level accepted for the definition of iodine deficiency, i.e. a concentration of less than 100 µg/l. The proportion of children having normal range (>100 µg /l) were 76.7%. Children with mild, moderate and severe grades of UIEL were 7.8%, 5.5% and 10.0% respectively. Prevalence of iodine deficiency in our study, calculated by proportion of children having UIEL of <100 µg/l, was 23.3%. Based on these values the area would be categorized as having “No iodine deficiency”.
Various studies done in India found prevalence of urinary iodine deficiency (i.e. MUIL <100 µg/l) in the range of 7.4% in Haryana [11] to 85.7% in Bhubaneshwar, Odisha [12].
Our results are similar to studies done in West Bengal (Chandra et al, 2006) and in Meerut District, UP [13].
In Bharuch district, 56.4% samples were found to have a Urinary Iodine Excretion (UIE) level of 100 µg/l or more, while 25.5% of the samples showed UIE levels between 50 and 99.9 µg/l, 13% between 20 and 49.9 µg/l and 5% below 20 µg/l. Thus, a mild deficiency was found in 25.5% of the children, moderate in 13%, and severe in 5% of the children [14].
In a study conducted in Ambala, Haryana, similar observations to the present study were made where the median urinary iodine excretion in the study sample was = 100 µg/l [15].
The age-wise distribution of urinary iodine showed that the proportion of children with urinary iodine excretion level of <20 µg/l was nil in 6 years age group while it was 18.2% in 9-10 years age group. A study conducted in Gujarat, showed that the proportion of children with urinary iodine excretion of <100 µg/l was lowest in the 6-year age group, whereas the highest proportion was found in the 10-year age groups 17. These results were in consonance with our findings (Table 4).
S. No.
Age (Years)
Urinary Iodine Excretion Level (µg/l)
Total
(%)
<20
(%)
20- 49.9
(%)
50-99.9
(%)
= 100
(%)
1.
6
0
(0.0)
0
(0.0)
1
(14.3)
6
(85.7)
7
(7.8)
2.
7-8
3
(10.3)
1
(3.4)
2
(6.9)
23
(79.3)
29
(32.2)
3.
9-10
2
(18.2)
3
(27.3)
1
(9.1)
5
(45.5)
11
(12.2)
4.
11-12
4
(9.3)
1
(2.3)
3
(7.0)
35
(81.4)
43
(47.8)
Total
9
(10.0)
5
(5.5)
7
(7.8)
69
(76.7)
90
(100.0)
Table 4: Distribution of urinary iodine excretion levels in different age groups.
The gender-wise distribution of urinary iodine excretion showed that the proportion of children with urinary iodine excretion >100 µg/l was slightly higher in females (82.8%) as compared to males (65.6%) (Figure 1). However in a study conducted in Gujarat it was almost similar among both the genders [16]. Proportion of the children below < 100 µg/l was slightly higher in females than males in a study conducted in Rajasthan [17].
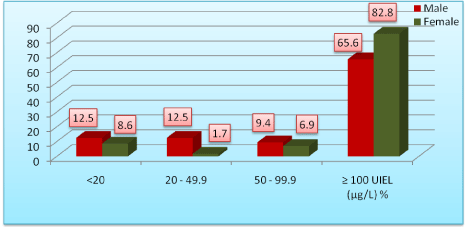
Figure 1: Distribution of urinary iodine excretion levels and sex.
As shown in (Figure 2), UIEL of <20 µg/l was highest in periurban areas (20.0%) followed by rural areas (15.4%). UIEL of =100 µg/l was seen maximum in students of urban areas (79.2%) while 69.2% of students had UIEL of =100 µg/l in rural and 60.0% in periurban areas.
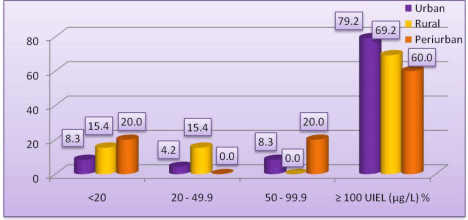
Figure 2: Distribution of urinary iodine excretion levels and area of residence.
In a study carried out in Bardhaman, 56.6% of urban children and 51.1% of rural children were biochemically iodine repleted and had Urinary Iodine Excretion (UIE) levels =100 µg/l. This indicates that more rural population was iodine deficient and this conforms to the results of present study [18]. Similar pattern was seen in school children in whom urinary iodine levels revealed that 95, 69.8 and 64.4% of the children from urban, rural and urban slum areas, respectively had UIE levels of more than 10 µg/dl [19].
UIEL of <20 µg/l was seen mostly in vegetarian students (20%) as compared to non vegetarian students (5%). In non-vegetarian students UIEL of 50-99.9 µg/l was higher than students with vegetarian dietary habits. Similar preponderance of non-vegetarian student was observed in students who had =100 µg/l UIEL (Figure 3).
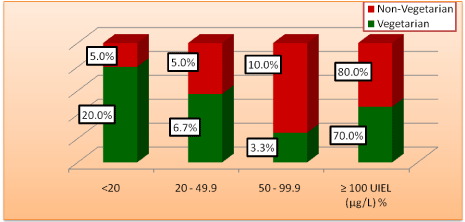
Figure 3: Distribution of urinary iodine excretion levels and dietary habit.
In a study conducted in Kochi opposite results were found. It was observed that vegetarians had higher urine iodine levels than nonvegetarians, but the number of vegetarians (n=18) was very small to make any valid comparison [20]. Other reason could be the intake of sea vegetations which are rich in iodine.
As it is evident from (Table 5), out of 13 students who were consuming salt with nil iodine content, 46.2% had <20 μg/l UIEL. As the iodine content of salt increased, UIEL also increased in most of the study population. This indicates that through intake of adequately iodized salt consumption, iodine nutrition can be improved.
S. No.
Iodine content (ppm)
Urinary Iodine Excretion Level (µg/l)
Total (%)
<20
(%)
20 - 49.9
(%)
50 - 99.9
(%)
= 100
(%)
1.
0
6 (46.2)
2 (15.4)
1 (7.7)
4 (30.8)
13 (14.4)
2.
1-7
2 (10.5)
1 (5.3)
3 (15.8)
13 (68.4)
19 (21.1)
3.
8 -- 15
1 (2.4)
0 (0.0)
1 (2.4)
39 (95.1)
41 (45.6)
4.
>15
0 (0.0)
2 (11.8)
2 (11.8)
13 (76.5)
17 (18.9)
Total
9
(10.0)
5
(5.5)
7
(7.8)
69
(76.7)
90 (100.0)
Table 5: Distribution of Urinary Iodine Excretion levels and iodine content of salt.
A statistically significant inverse association between the variable salt iodine content and urinary iodine excretion level was also found in a study conducted in Brazil. Iodine levels in salt for consumption were below the regulated of the Brazilian Health Ministry and they also detected a significant iodine deficiency in the children’s urine [21].
Conclusions
Urinary iodine is the most important biochemical indicator for current state of iodine nutrition because 90% of the ingested iodine is excreted in the urine [22]. A population is defined as free from iodine deficiency with a median UIE level of 100 μg/l and above, i.e. at least 50% of the urine samples had to have UIE level of above 100 μg/l and the percentage of sample with UIE level of < 50 μg/l should not be more than 20% [23]. In the present study, Median Urinary Iodine Excretion Level (MUIEL) for all children was found to be 140 μg/l, and 76.7% of them had UIE level of more than 100 μg/l with only 15.5% had UIE level of < 50 μg/l. Based on these values, the area would be categorized as having “No iodine deficiency”.
As the iodine content of salt increased, UIEL also increased in most of the study population. This indicates that through intake of adequately iodized salt consumption, iodine nutrition can be improved.
A missionary approach is required with effective and efficient coordination amongst all stakeholders of IDD control efforts in India especially in socio-economically backward areas to achieve and sustain the IDD control campaign.
At last, it is concluded that if all salt is iodized adequately and all families use only iodized salt, then iodine deficiency will no longer threaten the health and development of children. This will help building a healthy society and nation.
Integrated package of communication activities aimed at wholesalers, retailers and consumer with intensive social mobilization activities for a period of at least three years.
Proper Laboratory facilities for Urinary Iodine Excretion estimation as well as for checking the iodine content of salt through iodometric titration need to be set up in Aligarh District.
Better monitoring of iodine content in salt at various levels by the concerned agencies like, Food Safety Standard Authority of India (FSSAI) should be done.
Acknowledgement
We are thankful to Dr. Nafis Faizi, Assistant Professor, Department of Community Medicine, J.N.Medical College, A.M.U., Aligarh for pointing out few necessary corrections and spelling errors and sparing time for proof reading.
Contribution of Authors
First author did acquisition of data and analysis and interpretation of data Second author developed the concept and design of study and helped drafting the article and revising it critically for important intellectual content.
References
- de Benoist B, McLean E, Andersson M, Rogers L. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008; 29: 195–202.
- Department of Health and Family Welfare. Annual Report 2010-2011. New Delhi: Ministry of Health and Family Welfare, Government of India. 2011.
- National Iodine Deficiency Disorders Control Programme (NIDDCP). Revised Policy Guidelines, New Delhi: DGHS, Ministry of Health and Family Welfare, Government of India. 2006.
- World Health Organization. Vitamin and Mineral Nutrition Information System (VMNIS): Database on iodine deficiency, World Health Organization 2007.
- Anand K, Pandav CS. Iodination of Irrigation water. The National Medical Journal of India. 1996; 9: 227-278.
- Directorate General of Health Services, Govt. of India. National Goitre Control Programme. Prevalence rate of goitre according to survey conducted in areas during. 1981-2003. 1995.
- Rao PSSS, Richard J. Introduction to Biostatistics and Research Methods. 4th Edition, P H I Learning Private Limited, New Delhi. 2009; 195.
- WHO/UNICEF/ICCIDD. Indicators for assessing iodine deficiency disorders and their control through salt iodization WHO/NUT/94.6 Geneva: WHO; 1994.
- Dunn JT, Crutchfield HE, Gutekunst R, Dunn D. Methods for measuring iodine in urine. A Joint Publication of WHO/ UNICEF/ICCIDD. 1993; 18-23.
- World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: WHO. 2007.
- Kapil U. Urinary iodine excretion levels amongst school children in Haryana. Indian Pediatrics. 2009; 46: 57-59.
- Sethy PGS, Bulliyya G, Mallick G, Swain BK, Kar SK. iodine deficiency in urban slums of Bhubaneswar. Indian Journal of Pediatrics. 2007; 74: 917- 921.
- Kapil U, Singh JV, Tandon M, Pathak P, Singh C, Yadav R. Assessment of iodine deficiency disorder in Meerut District, Uttar Pradesh. Asia Pacific J Clin Nutr. 2000; 9: 99-101.
- Chandwani HRK, Shroff BD. Prevalence of goiter and urinary iodine status in six-twelve-year-old rural primary school children of Bharuch District, Gujarat, India. Int J Prev Med. 2012; 3: 54–59.
- Chaudhary C, Pathak R, Ahluwalia SK, Goel RKD, Devgan S. Iodine deficiency disorder in children aged 6-12 years of Ambala, Haryana. Indian Paediatrics 2013; 50: 587-589.
- Damor JR, Padhiyar NG, Ninama GL. Urinary iodine excretion in urine samples among children in Dahod District, Gujarat. Indian Journal of Clinical Practice 2013; 23: 560-564.
- Singh MB, Marwal R, Lakshminarayana J. Assessment of iodine deficiency disorders in school age children in Jodhpur District of Rajasthan. Journal of Human Ecology. 2010; 32: 79-83.
- Sen S, Mondal A, Dasgupta A, Chakraborty I. Prevalence of iodine deficiency disorders among schoolchildren in three blocks of Bardhaman District and Bardhaman Municipal Area of West Bengal, India: a comparative study. Southeast Asian Journal of Tropical Medicine Public Health. 2005; 36: 1321- 1324.
- Kapil U, Shah AD, Bhasin SK, Singh C, Balamurugan A, Prakash S, et al. Iodine content of salt consumed and iodine status of school children in Delhi. Indian Pediatrics 1996; 39: 586-586.
- Menon VU, Chellan G, Sundaram KR, Murthy S, Kumar H, Unnikrishnan AG, et al. Iodine status and its correlations with age, blood pressure, and thyroid volume in South Indian women above 35 years of age (Amrita Thyroid Survey). Indian Journal of Endocrinology and Metabolism. 2011; 15: 309-315.
- Nimer M, Silva ME, de Oliveira JED. Relationship between iodized salt and urinary iodine excretion in school children, Brazil. Rev Saude Publica. 2002; 36: 500-504.
- Zimmermann MB. Iodine requirements and the risks and benefits of correcting iodine deficiency in population. J Trace Elem Med Biol. 2008a; 22: 87-89.
- WHO/UNICEF/ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. France: World Health Organization; 2007. 47-51.
