Abstract
Background and Objectives: Several factors affect the uptake and utilization of iodine in the body. The goitrogenic substances are present in cabbage, cauliflower, turnip, lady’s finger, sweet potato, cassava etc. affect the metabolism of iodine. The present study was conducted to find out the prevalence of goitre in school children and its association of dietary factors with goitre.
Methods: From 1st to 5th standard children (age group 6–12 years) were the “sampling units.” The required sample was selected by “Multistage sampling” by doing a sub-sampling. The sample size (N) calculated was 879. However, a total of 950 participants were included in the study.
Results: Out of 950 students, only 17 (1.8%) had goiter. Higher numbers of students affected with goitre were vegetarians (2.2%) as compared to nonvegetarians (1.6%). Dietary history of goitrogen intake was present in 91.3% of the study population. Majority of the students (52.8%) took the vegetables containing goitrogenss regularly (2-3 times) per week. Prevalence of goitre in children taking goitrogens was slightly higher. Lowest prevalence of goitre (0.6%) was seen in students taking ›15 ppm iodine salt.
Conclusions and Recommendations: The findings suggest that there might be goitrogens or other dietary factors playing a role besides iodine deficiency. Presence of goitre reflects the iodine deficiency in the past. The students suffering from goitre could have taken non-iodised salt in the past and they might have started taking iodised salt (›15 ppm) recently. Sustained IEC (Information, Education and Communication) activities should be carried out more vigorously.
Keywords: Iodine deficiency; Goiter; Goitrogens; Iodised salt
Introduction
“Iodine deficiency is so easy to prevent that it is a crime to let a single child be born mentally handicapped for the reason.” H. Labouisse; Executive Director, UNICEF (1978), Iodine deficiency is one of the most neglected and widespread of all nutritional deficiencies, constituting a brake on human development. Iodine is required for the synthesis of thyroxine (T4) and triiodothyronine (T3). These hormones are very important in the regulation of the metabolism of proteins, carbohydrates and fats and almost all the activities of the body.
Two most recognized features of iodine deficiency in the past were endemic goitre and cretinism. Certainly there are other consequences of iodine deficiency affecting all age groups including foetus. Goitre is indeed the visually obvious and familiar feature of iodine deficiency but understanding of the other consequences of iodine deficiency has greatly expanded in the last 25 years so that it is not surprising that the wider designation of “IDD” is now considered more appropriate [1].
In India, IDD has been identified as a public health problem. It has been observed that the world’s most intense goitre belt is in India stretching 2400 Kms from Kashmir in the North West to the Naga Hills in the East. In addition to the known Himalayan endemic belt, iodine deficiency and endemic goitre has been reported from many other states in the country as well. New pockets of iodine deficiency are being identified continuously. Surveys conducted in India have revealed that out of the 325 districts surveyed in India, 263 districts are IDD-endemic, i.e. the prevalence of IDD is above 10 per cent in the population [2]. Out of total population of India (approx 1200 million) more than 200 million are at risk of IDD [3]. WHO has recommended that for assessment of Iodine deficiency in an area, children in the age group 6-12 years should be surveyed [4].
According to the World Health Organization (WHO), iodine deficiency occurs in 130 countries in the world, and 2.2 billion people live in iodine-deficient areas [5]. Surveys conducted in India have revealed that out of the 325 districts surveyed in India, 263 districts are IDD-endemic, i.e., the prevalence of Iodine Deficiency Disorder (IDD) is above 10% in the population [6].
Several factors affect the uptake and utilization of iodine in the body. The importance of dietary and environmental factors is well documented. The goitrogenic substances are present in cabbage, cauliflower, turnip, lady’s finger, sweet potato, cassava etc. and affect the metabolism of iodine. It is believed that these may possibly play a contributory role in the causation of endemic goitre.
At present best source for iodine supplementation is iodinated salt in the form of “Iodised Salt” containing potassium iodide (KI) and “lodated Salt” containing potassium iodate (KIO3). KIO3 has an advantage over KI in that it is more stable and has a longer shelf life.
The other methods of iodine supplementation are injection of iodised oil, addition of iodine to bread and iodination of irrigation water but these methods are not applicable to all the people [7].
After trying many methods, salt emerges as the best vehicle for iodine supplementation. After iodisation of salt, it is to be made mandatory that it should contain 30 parts per million (ppm) iodine at the manufacturer level and 15 ppm at distribution channel including consumer level.
If salt with recommended level of iodine is consumed by a community for just 12 months, no more cretins will be born, no more babies will suffer from retarded physical or mental development attributed to iodine deficiency.
The present study was conducted with the following Objectives:
1. To find out the prevalence of goitre in school children.
2. To find out the association of dietary factors with goitre.
Materials and Methods
The study was conducted among school children (6-12 years). Three government and four private schools in Aligarh were selected.
Sampling unit
1st to 5th standard children of the schools (age group 6-12 years) were the “sampling units” for study conducted in schools. This is the preferred group as it is usually accessible. There is a practical reason for not measuring very young age groups. The smaller the child, the smaller the thyroid and it is more difficult to perform palpation [4]. In the selected schools, almost every child of 1st standard had completed six years of age and most of the children of 5th standard were completing twelve years of age.
Sample size
From 1st to 5th standard children (age group 6-12 years) were the “sampling units.” Directorate General of Health Services found a goiter prevalence rate of 12% in Aligarh District [8]. Taking “p” as 12% and relative error (l) 20% of “p,” the sample size (N) was calculated as [9]:
N = 4 × P × q/l2
q (%) =100 - p,
N = 4 × 12 × 88/(0.2 × 12)2
N = 733
Taking into consideration 20% non response/non-cooperation rate, total sample size was:
N = 733+ (0.2 × 733)
N = 879
However, a total of 950 participants were included in the study.
Plan of study
Schools were contacted several days before the study began to inform the principals of the schools, the study purpose and to get consent from them as well as parents/guardians of children. Schools were contacted several days before the study began to inform the principals of the schools, the study purpose and to get consent from them as well as parents/guardians of children. In consultation with principal, a suitable date (a day on which the attendance in the school was maximum, preferably early in the week, avoiding national and state holidays), time and place for interviewing and examining the children were chosen. As a part of ethical considerations, they were briefed about the presentation of IDD, and its consequences and methods available for its prevention especially health benefits of taking iodized salt in diet, food items which prevent the utilization of iodine in the body. This helped us having their maximum participation for conducting the study in school children, and it also ensured good attendance of students.
The school authorities were asked to provide us the list of students who were enrolled in classes from 1st to 5th standards and were in the age group 6-12 years. We requested for school records showing their dates of births. The age was classified according to their dates of births.
Sampling procedure
The required sample was selected by “Multistage sampling” by doing a subsampling. In the first stage, schools were selected over a period of time, and permission was obtained from school authorities. In the second stage, a list of students in class 1st-5th standard in the age of 6-12 years was obtained. Our “sampling frame” consisted of number of students selected from one school. As per probability proportional to size method, number of students in a school was proportional to the strength of a total number of students (6-12 years) from all schools. The next stage was to select students in a school. With the help of random number table, a random sampling method was applied to select the final numbers of students from a school to be included in the study. We assigned each student a serial number in that school.
Every child was asked to bring approximately two teaspoons (10g) of salt in labeled auto sealed polythene pouches.
Inclusion criteria
1. Students of schools whose principals gave consent to our study.
2. Students of classes from 1st to 5th standard who were of age group 6-12 years.
3. Students whose parents/guardians gave consent to our study.
Exclusion criteria
1. Students not attending the school on the day of study.
2. Students whose parents/guardians did not give consent to our study.
3. Students aged less than 6 years and more than 12 years.
Each child was examined clinically for the presence of goitre as per the standard WHO guidelines and goitre was graded according to the classification given below [4]:
Grade 0: No palpable or visible goitre
Grade 1: A goitre that is palpable but not visible when the neck is in the normal position (i.e., the thyroid is not visibly enlarged).
Thyroid nodules in a thyroid which is otherwise not enlarged fall into this category
Grade 2: A swelling in the neck that is clearly visible when the neck is in a normal position and is consistent with an enlarged thyroid when the neck is palpated
Salt testing
The iodine content of salt samples was tested using the Spot Testing Kit (STK) in the school in front of students and teachers, in halls/schools of residence of Aligarh Muslim University, and in the community. This method is similar with National Family Health Survey estimates conducted in the year 2005-06 [10].
Approval of Institutional Ethics and Research Advisory Committee, Faculty of Medicine, J.N. Medical College, A.M.U., Aligarh was also taken.
Those found to have goiter and other diseases were referred to nearby health centers or J.N. Medical College Hospital for further evaluation and management.
Institutional Ethics Committee approved the study. Statistical analysis was done using IBM SPSS version 20. Chi-square was used to find out association and ’p’ value of ‹ 0.05 was considered as significant.
Results
Figure 1 shows that out of 950 students, only 17 (1.8%) had goitre and 933 (98.2%) students did not have goitre. Out of 17 students having goitre, 16 (1.8%) had Grade 1 goitre and only 01 (0.1%) student was suffering from Grade 2 goitre.
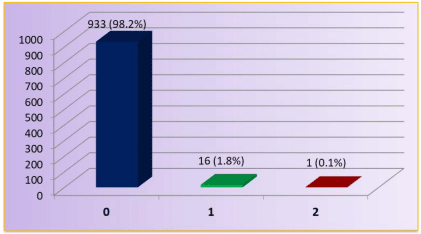
Figure 1: Grades of goitre in the study population.
Figure 2 shows Distribution of study population according to goitrogens intake.
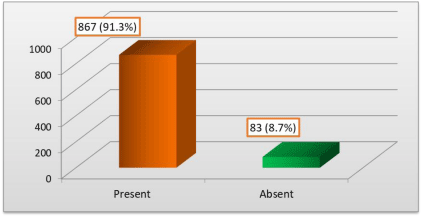
Figure 2: Distribution of study population according to goitrogens intake.
Table 1 shows that slightly higher numbers of students affected with goitre were vegetarians (2.2%) as compared to non-vegetarians (1.6%).
S. No.
Diet
Goitre
Total
Present
Absent
No.
%
No.
%
No.
%
1
Vegetarian
7
2.2
306
97.8
313
32.9
2
Non-Vegetarian
10
1..6
627
98.4
637
67.1
Total
17
1.8
933
98.2
950
100
Table 1: Distribution of study population according to dietary habits and goiter.
Table 2 shows the intake of vegetables and other food items containing goitrogens. It was observed that more than one type of food item containing goitrogen was taken by most of the students and most commonly consumed food items were cabbage, cauliflower and lady’s finger, 307 (35.4%).
S. No.
Food items containing goitrogens (n=867)
No.
%
1.
Cabbage (1)
23
2.7
2.
Cauliflower (2)
14
1.6
3.
Turnip (3)
3
0.3
4.
Lady's Finger (4)
64
7.4
5.
Maize (5)
1
0.1
6.
Others (6)
2
0.2
7.
1+2+3
53
6.1
8.
4+5
15
1.7
9.
1+2+3+4+5
70
8.1
10.
1+2+4
307
35.4
11.
3+4
8
0.9
12.
1+2+4+5
8
0.9
13.
1+2+3+4
48
5.5
14.
1+5+6
3
0.3
15.
1+4
21
2.4
16.
2+4
34
3.9
17.
1+2
40
4.6
18.
2+3+4
3
0.3
Table 2: Distribution of study population according to intake of food items containing goitrogen.
Figure 3 presents the frequency of intake of vegetables containing goitrogens. Out of 867 students taking goitrogens in their diets, majority of the students 458 (52.8%) took these vegetables regularly (2-3 times) per week. 294 (33.9%) of the subject used to take these vegetables frequently (once a week) and only 115 (13.3%) of the students consumed these vegetables occasionally (once a fortnight).
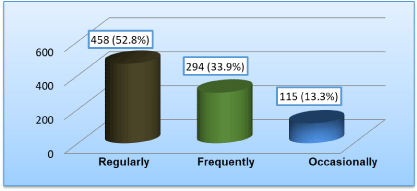
Figure 3: Distribution of study population according to frequency of
goitrogens intake.
We can see from Table 3 that the prevalence of goitre among children taking goitrogen was slightly higher (2.0%) as compared to those not taking them (0.0%) but the difference was insignificant.
S. No.
Goitrogen intake
Goitre
Total
Present
Absent
No.
%
No.
%
No.
%
1
Present
17
2
850
98
867
91.3
2
Absent
0
0
83
100
83
8.7
Total
17
1.8
933
98.2
950
100
Table 3: Distribution of study population according to goitrogen intake & goiter.
As given Table 4 out of 875 students who were consuming powdered salt, goitre was present in 1.5% of the study subjects. In case of crystalline salt, higher prevalence of goitre (4.9%) was observed. When presence of goitre was compared in powdered and crystalline salt consuming students, a statistically significant difference was found significant (p ‹.05).
S. No.
Goitre
Type of Salt
Total
Powdered
Crystalline
Both
No.
%
No.
%
No.
%
No.
%
1
Present
13
1.5
3
4.9
1
7.1
17
1.8
2
Absent
862
98.5
58
95.1
13
92.9
933
98.2
Total
875
92.1
61
6.4
14
1.5
950
100
Table 4: Distribution of students with goitre and type of salt consumed.
Table 5 shows that lowest prevalence of goitre (0.6%) was seen in students taking ›15 ppm iodine salt where as prevalence was high in those taking salt with nil iodine (2.5%) but the difference was insignificant.
S. No.
Goitre
Iodine Content (ppm)
0
7-Jan
15-Aug
>15
No.
%
No.
%
No.
%
No.
%
1
Present
5
2.5
6
1.9
5
1.6
1
0.6
2
Absent
197
97.5
310
98.1
270
98.4
156
99.4
Total
202
100
316
100
275
100
157
100
Table 5: Distribution of students with goitre and quality of salt consumed.
Discussion
In the present study, the total goitre rate (TGR) or prevalence was 1.8%. Similarly, in the state of Jharkhand, very low prevalence of goitre (0.9%) was noticed [11]. Very low prevalence of 0.02% was recorded in Bishnupur, Badaun districts in a study conducted during 1997-2000 [12]. The goitre prevalence in a study population was found to be 0.125% in Bellur Hobli in the southern part of India and 4.29% in Mahasamund [13,14]. The total goitre rate was 4.83% among primary school children aged 6-12 years in Jamnagar [15]. However, higher prevalence was also observed in another study where TGR was 7.75% [16]. Similar prevalence rates were also found in other studies [17,18]. The findings were also high (11.8%) as the study was conducted in Sub-Himalayan Jammu region [19].
Similar observations were made in a study conducted in Kottayam, Kerala where children who had consumed vegetables frequently showed higher prevalence of goitre than non-vegetarians [20]. There was significant association found between prevalence of goitre and vegetarian diet in a study conducted in Dehradun [21].
However, researchers found that people following non vegetarian food habit are most heavily affected with thyroid disease in Ernakulam City and Cherthala Town of Kerala State, India [22].
Most of these studies were focused on projecting the devastating effects of various goitrogenic substances, taken as staple food, however such food taken more than three times a week has significant effect. Effect of cooking on goitrogens has not been well established yet. This area needs further research. WHO recommends that the amounts and frequency of intake of such goitrogenic food is to be discussed with the healthcare practitioner and also work up the most suitable combinations which the physicians often tend to forget. They do not have time to discuss these issues in their clinics. Hence it is important to educate patients on benefits of good and rightful diet along with its quantity to avoid possible goitrogenic effects [23].
It has been hypothesized that goitrogens could induce goitre formation [23]. Researchers identified goitrogens in Brassica family (Cruciferous vegetables including cabbage, cauliflower, turnip), similar to these food items taken by children in our study [24]. In a goitre prevalence study among school children conducted in West Bengal in India, consumption of goitrogens in the region was the major etiological factor in elevated goitre prevalence (38%) in spite of adequate iodine intake [25]. Similar observations were also made in another study [26].
A direct correlation was also seen between the frequency of cabbage intake and goitre prevalence. Children who took cabbage regularly were exposed to goitre more than those who had never taken cabbage [27]. However, in a study conducted in Sri Lanka, dietary goitrogens showed no significant association with the prevalence of goitre [28].
Presence of goitre reflects the iodine deficiency in the past. The students suffering from goitre could have taken non iodised salt in the past and they might have started taking iodised salt (›15 ppm) recently. Other contributory factors like environmental and dietary factors could also be implicated in the causation of goitre. Persistence of endemic goitre after adequate iodization has been observed by many authors all over the world, similar to our observation [29].
High prevalence of goitre (18.1%) was found in the Narmada District where 12.4% of grade 1 and 5.7% of grade 2 was noticed in the study subjects in-spite of 93% households were using iodized salt with ›15 ppm iodine [30].
Similar results were also found in a study conducted in Chandigarh where use of iodised salt was reported in families of 149 (84.2%) children with goitre and 126 (83.4%) control children [31- 39].
Conclusion
Several factors affect the uptake and utilization of iodine in the body. The importance of dietary factors in causation of iodine deficiency is well documented. The goitrogenic substances, present in cabbage, cauliflower, turnip, lady’s finger, sweet potato, cassava etc. affect the metabolism of iodine. The findings of the present study suggests that there might be goitrogens or other dietary factors along with environmental factors playing a role besides iodine deficiency that need to be explored. Lowest prevalence of goitre was seen in students taking ›15 ppm iodine salt. Presence of goitre reflects the iodine deficiency in the past. The students suffering from goitre could have taken non iodised salt in the past and they might have started taking iodised salt (›15 ppm) recently. Sustained IEC (Information, Education and Communication) activities should be carried out more vigorously so that people are made aware about the spectrum of iodine deficiency disorders and its prevention by consuming salt iodated adequately (iodine content › 15 ppm). For current iodine status in the surveyed population, Urinary Iodine Excretion Level (UIEL) estimation is recommended.
Acknowledgement
We are thankful to students, our study population for giving their consent to get the study conducted.
References
- Hetzel BS. Iodine deficiency disorders (IDD) and their eradication. Lancet. 1983; 2: 1126-1129.
- Ministry of Health and Family Welfare, Government of India. New Delhi. Department of Health and Family Welfare. Annual Report. 2010-2011.
- Directorate General of Health Services, Govt. of India, New Delhi. National Goitre Control Programme. Prevalence Rate of Goitre According to Survey Conducted in Areas During 1981-2003.
- WHO. Vitamin and Mineral Nutrition Information System (VMNIS): Database on Iodine Deficiency, World health organization. 2007.
- De Benoist B, McLean E, Andersson M, Rogers L. Iodine deficiency in 2007: Global progress since 2003. Food Nutr Bull. 2008; 29: 195-202.
- Directorate General of Health Services, Govt. Of India, New Delhi. National Goitre Control Programme. Prevalence Rate of Goitre According to Survey Conducted in Areas During 1981-2003.
- Anand K, Pandav CS. Iodination of Irrigation water. The National Medical Journal of India. 1996; 9: 227-278.
- Rao PS, Richard J. Introduction to Biostatistics and Research Methods. 4th edn. New Delhi: P H I Learning Private Limited. 2009.
- National Family Health Survey (NFHS-3) 2005–06. International Institute for Population Sciences (IIPS) and Macro International, 2007. Volume I. Ministry of Health and Family Welfare, Government of India. Mumbai: IIPS.
- Patro BK, Saboth P, Zodpey S, Shukla A, Karmakar MG, Pandav CS. Tracking Progress Toward Elimination of Iodine Deficiency Disorders in Jharkhand, India. Indian J Community Med. 2008; 33: 182-185.
- Toteja GS, Singh P, Dillon BS, Saxena BN. Central Co-ordinating unit Kashmir (India): IDD in 15 districts of India. Indian Journal of Pediatrics. 2004; 7: 25-28.
- Sridhar PV, Kamala CS. Iodine Status and Prevalence of Goitre in School Going Children in Rural Area. Journal of Clinical Diagnosis and Research. 2014; 8: 15-17.
- Sinha AK, Sharma H, Panda PS, Chandrakar A, Pradhan SK, Dixit S. Prevalence of goitre, iodine uptake and salt iodization level in Mahasamund district of Chhattisgarh: a baseline study in Central India. International Journal of Research in Medical Sciences. 2016; 4: 3590-3594.
- Makwana NR, Shah VR, Unadkat S, Shah HD, Yadav S. Goiter prevalence and current iodine deficiency status among school age children years after the universal salt iodization in Jamnagar District, India. Thyroid Research and Practice. 2012; 9: 40-44.
- Rawal SV, Kedia G. A prevalence study of iodine deficiency disorder in children of primary schools in Gandhinagar District. National Journal of Community Medicine. 2011; 2: 478-482.
- Chudasama RK, Verma P, Banerjee A, Amin CD, Mahajan R. Current iodine status and progress over the last decade towards elimination of iodine deficiency in Rajkot District, Gujarat. Online Journal of Health and Allied Sciences. 2009; 8: 1-5.
- Pandav CS. Executive summary: Tracking progress towards sustainable elimination of iodine deficiency disorders in Orissa. IQ Jagriti. 2005; 3: 8-9.
- Bhat IA, Pandit IM, Mudassar S. Study on prevalence of iodine deficiency disorder and salt consumption patterns in Jammu region. Indian Journal of Community Medicine. 2008; 33: 11-14.
- Gur E, Ercan O, Can G, Akkus S, Guzeloz S, Ciftcili S, Arvas A, Iltera O. Prevalence and risk factors of iodine deficiency among school children. J Trop Pediatr. 2003; 49: 168-171.
- Hetzel BS, Dunn JT, Stanbury JB. The prevention and control of iodine deficiency disorders. Elsevier Science Publishers, Biochemical Division. Amsterdam. 1987.
- Misra S, Kantharia SL, Damor JR. Prevalence of goitre in 6 -12 years schoolgoing children of Panchmahal District in Gujarat, India. Indian J Med Res. 2007; 126: 475-479.
- Sharma U, Sharma JP, Sharma A, Kumar A, Shukla N. Prevalence of goitre among school going children in urban area of Dehradun. International Journal of Research in Medical Sciences. 2015; 3: 198-200.
- Kamath R, Bhat V, Rao RSP, Acharya D, Kapil U, Kotian MS, et al. Prevalence of goitre among school children in Belgaum District. Indian Journal of Pediatrics. 2009; 76: 825-828.
- Sahu T, Sahani NC, Satpathy DM, Behera TR. Prevalence of goiter in 6-12 year old children of Kandhamal District in Orissa. Indian Journal of Community Medicine. 2005; 30: 51-52.
- Biswas AK, Chakraborty I, Das DK, Chakraborty A, Ray D, Mitra K. Elimination of iodine deficiency disorder-current status in Purba Medinipur District of West Bengal, India. Indian Journal of Public Health. 2008; 52: 130-135.
- Moorthy D, Patro BK, Das BC, Sankar R, Karmakar MG, Pandav CS. Tracking progress towards sustainable elimination of iodine deficiency disorders in Orissa. Indian Journal of Public Health. 2007; 51: 211-215.
- Chandra AK, Bhattacharjee A, Malik T, Ghosh S. Goiter prevalence and iodine nutritional status of school children in a Sub-Himalayan Tarai Region of Eastern Uttar Pradesh. Indian Pediatrics. 2008; 45: 469-474.
- Ramesh, Parameshwari P, Manjula V D, Shobha A, Ajith R. Prevalence of goitre among school children in Kottayam, Kerala. International Journal of Medical and Health Sciences. 2013; 2: 331-336.
- James R, Kumar VTV. Study on the prevalence of thyroid diseases in Ernakulam City and Cherthala Town of Kerala State, India. International Journal of Scientific and Research Publications. 2012; 2: 1-3.
- Bindu MK, Mandava K, Miriyala M. Effects of dietary goitrogens on hypothyroidism. Annals of Drug Discovery and Biomedical Research. 2014; 1: 1-4.
- Gaitan E. Goitrogens in food and water. Annual Review of Nutrition. 1990; 10: 21-37.
- Fenwick GR, Heaney RK. Glucosinolates and their breakdown products in cruciferous crops, foods and feeding stuffs. Food Chemistry 198.3; 11: 249- 271.
- Chandra AK, Singh H, Tripathy S, Debnath A, Khanam J. Iodine nutritional status of children in North East India. Indian Journal of Pediatrics. 2006; 73: 795-798.
- Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect 2002; 110: 349-353.
- Mezgebu Y, Mossie A, Rajesh PN, Beyene G. Prevalence and severity of iodine deficiency disorder among children 6-12 years of age in Shebe Senbo District, Jimma Zone, Southwest Ethiopia. Ethiopian Journal of Health Sciences. 2012; 22: 196-204.
- Fernando R, Pinto MDP, Pathmeswaran A. Goitrogenic food and prevalence of goitre in Sri Lanka. International Journal of Internal Medicine. 2012; 1: 17- 20.
- Filteau SM, Sullivan KR, Anwar US, Anwar ZR, Tomkins AM. Iodine deficiency alone cannot account for goitre prevalence among pregnant women in Modhupur, Bangladesh. European Journal of Clinical Nutrition. 1994; 48: 293-302.
- Pandor JM, Damor JR, Padhiyar NG, Ninama GL. A study to measure prevalence of goiter in schoolchildren of Narmada District, Gujarat. National Journal of Community Medicine. 2011; 2: 201-203.
- Das S, Bhansali A, Dutta P, Aggarwal A, Bansal MP, Garg D. Persistence of goitre in post iodization phase: micronutrient deficiency or thyroid autoimmunity. Indian Journal of Medical Research. 2011; 133: 103-109.
