Abstract
This study aimed to assess why and at what point along the care journey patients tend to discontinue a real-world GLP-1 RA-supported Digital Weight-Loss Service (DWLS) for women in the UK. To achieve these objectives, a cohort of 8294 patients was retrospectively analyzed, using both descriptive statistics and correlation tests. Mean adherence to the program was 183.1 (±148.9) days and the most common reasons for discontinuation were program cost (38.7%), dissatisfaction with results (16.8%), intolerable side effects (15.4%), attainment of one’s weight-loss goal (7.3%) and temporary pause/holidays (5.6%). Patients who were older, Caucasian and Overweight tended to adhere to the program for a statistically significantly longer period than their younger, non-Caucasian and higher BMI-class peers. This study provides important foundational insights to the scarce literature on real-world GLP-1 RA-supported DWLSs, which are becoming increasingly popular in the obesity epidemic.
Introduction
Obesity is a chronic disease affecting over a quarter of the British population, with a further 37.9% of Brits estimated to be overweight [1]. In recent years, randomized controlled trials have demonstrated the unprecedented effectiveness of Glucose-like Peptide-1 Receptor Agonists (GLP-1 RAs) in inducing weight loss for people with overweight and obesity [2-4]. To access these medications in real-world settings, a growing number of people are using Digital Weight-Loss Services (DWLSs) [5]. Researchers have argued that in addition to helping overcome undesirable clinic waiting periods and travel times, DWLSs can facilitate care continuity and reduce patient perception of stigma [6,7]. However, evidence to support these views is scarce and most questions around the quality and safety of real-world DWLSs remain unanswered.
Major health institutions such as the World Health Organization (WHO) and the UK National Institute for Health and Care Excellence (NICE) emphasize the importance of specialist multidisciplinary care in the treatment of obesity, given the disease’s complexity and chronic nature [8,9]. A factor that has historically limited the effectiveness of such care in a weight-loss context is program adherence [10]. While the majority of research is limited to Face-to-Face (F2F) interventions, there is no evidence to corroborate the logic that delivering weight-loss programs through digital modalities improves adherence. Scholars have suggested that certain DWLSs could observe lower adherence rates than F2F models as they are typically unsubsidized and expensive, which can lead to treatment cycling – an ill-advised phenomenon where patients cycle on and off their subscription and often increase their risk of weight gain and the development of comorbidities [11].
Another important consideration in the DWLS discussion is the broad quality spectrum. Services range from websites that offer little more than private prescriptions for GLP-1 RA medications to programs with coordinated Multidisciplinary Teams (MDTs) that guide patients through health coaching and GLP-1 RA therapy and are supported by robust clinical governance systems [12,13]. Although it is feasible that DWLSs at the more comprehensive end of this spectrum would observe higher adherence rates, a recent study on a cohort of patients of the digital GLP-1 RA-supported Juniper Australia program suggest otherwise [14]. The study found that only 21% of the cohort’s 10,918 patients attended their week-14 and 32 follow-up consultations.
This study aims to assess why and at what stage of the care journey patients of the Juniper UK weight-loss program tend to discontinue their subscription. The Juniper UK program allocates each patient an MDT consisting of a doctor, pharmacist, health coach and medical support officer who coordinate asynchronous care through an integrated digital platform.
All patient data, including questionnaire responses and clinician decisions around both GLP-1 RA therapy and health coaching, are stored in Eucalyptus’ (Juniper parent company) central data repository. As of 12 March, the Juniper program has cared for over 55,000 weight-loss patients across Australia, Germany, Japan and the UK, including over 17,500 British patients. This study’s results will play an important foundation in the obesity care literature by generating novel insights on patient adherence to real-world GLP-1 RA DWLSs.
Methods
The study conducted a retrospective analysis of a cohort of patients who commenced the Juniper UK weight-loss program between 28 April 2022 and 1 April 2023. To be included in the study, patients needed to receive a minimum of one order of Semaglutide and satisfy all criteria that prescribing doctors use to determine patient eligibility for the Juniper weight-loss program. These criteria follow the NICE guidelines for semaglutide weight-loss therapy, such as those concerning BMI thresholds, medicine contraindications and various comorbidity risks. All relevant study data were retrieved from Eucalyptus’ central data repository on Metabase.
3 discontinuation paths exist under the Juniper program (and likely most other GLP-1 RA-supported DWLSs). 1/ Patient notified discontinuation – when a patient notifies their MDT that they are pausing or stopping their subscription; 2/ Consult drop-out – when a patient fails to attend review or follow-up consult and does not communicate the reason within 50 days (a period long enough for patient to sustain GLP-1 RA order if they stretch their dosing schedule); and 3/ Doctor decision – a patient’s prescribing doctor decides to terminate the patient’s subscription for medical reasons. For the purposes of this study, it is important to add forth discontinuation category to capture the patients of the cohort who have not paused their subscription at any point and are still active users of the Juniper weight-loss program. Despite the ‘Still active’ status of this category, the date of analysis (12 March 2024) will be taken as their discontinuation date when calculating central tendency data. For categories 1 (patient-notified discontinuers) and 3 (doctor decision), the day the decision was communicated will be taken as the discontinuation date, while for consult drop-outs, a patient’s last scheduled consult (the one they failed to attend) will be used.
Patients who notified their MDT of their decision to discontinue their subscription were asked on the same day to give their main reason for discontinuing by their medical support officer. Consult drop-out patients were asked this question 50 days after their last attended follow-up or review consult. The question was re-sent to patients from both these categories if a response wasn’t received by their MDT within 72 hours. Patients whose subscriptions were cancelled by their doctor for medical reasons were not sent this question, as the discontinuation reason was clear.
The study’s coprimary endpoints are mean adherence days, i.e., the total days from program initiation to discontinuation, and the distribution of reasons for discontinuation. Sub-group analyses of the coprimary endpoints, including groups assembled by BMI category and discontinuation category, represent the study’s exploratory endpoints.
Results
8294 patients satisfied the study criteria and were included in the final analysis. Mean age of the cohort was 44.4 (±11.2) years, mean BMI was 35.2(±7.4) kg/m2, and 82.5% of patients were of Caucasian ethnicity (Table 1). Mean adherence to the Juniper weight-loss program was 183.1 (±148.9) days. Discontinuation reason data were taken from 3754 patients, of which 3716 were from the ‘patient notified MDT’ category and 38 from the ‘consult drop-out category’. The most common reason given for discontinuation was program cost (38.7%), followed by dissatisfaction with results (16.8%), intolerable side effects (15.4%), attainment of weight-loss goal (7.3%) and temporary pause/going on holidays (5.6%).
Demographic information
Age – mean (SD)
45.4 (11.2)
Sex at birth – no. (%)
Female (Juniper)
7670 (92.5)
Male (Pilot)
624 (7.5)
Ethnicity – no. (%)
Caucasian
6843 (82.5)
Asian (Inc. subcontinent)
586 (7.1)
Black African or African Caribbean
428 (5.2)
Not listed
255 (3.1)
Middle Eastern
114 (1.4)
Latino/Hispanic
68 (0.8)
Baseline clinical information – mean (SD)
BMI
35.2 (7.4)
Table 1: Patient characteristics.
Nearly two-thirds of the cohort’s patients notified their MDT of their decision to discontinue (62.6%) and mean adherence for this sub-group was 106.1 (±87.1) days. A comparable percentage of patients either dropped out of their consults (19.3%) or are still active users of the Juniper program (17.3%, with mean adherence of 219.9 (±116) days and 420.6 (76.1) days observed for those groups, respectively. Only 64 (0.8%) of patients had their subscriptions cancelled by their doctor due to medical reasons, recording a mean adherence period of 188.8 (±111) days. An Analysis of Variance (ANOVA) found that the mean difference between categories was statistically significant [F (3, 8290) = 4498, p <0.01] and a post hoc Tukey HSD test revealed that all four categories varied from one another to a statistically significant degree. A comparison of adherence distribution and central tendencies across the four discontinuation categories is presented in Figure 1. All mean adherence data and discontinuation reason data are presented in Tables 2 and 3, respectively.
Adherence period – mean (SD) days
Total cohort
183.1 (148.9)
Discontinuation categories
Patient notified MDT
106.1 (±87.1)
Consult drop-out
219.9 (±116)
Doctor cancelled
188.8 (±111)
Still active
420.6 (±76.1)
BMI categories – n patients
Overweight (24.5-29.99 kg/m2) - 1535
194.8 (±154)
Class 1 obesity (30-34.99 kg/m2) - 3490
185.7 (±151)
Class 2 obesity (35-39.99 kg/m2) - 1801
179.1 (±156)
Class 3 obesity (40 kg/m2 and over)- 1138
172.0 (±144)
Ethnicity categories – n patients
Caucasian – 3433
176.1 (±131)
Non-Caucasian – 728
155.7 (±122)
Not stated – 4133
194.6 (±166)
Table 2: Adherence period data.
Discontinuation reasons*
n (%)
Cost
1440 (38.3)
Dissatisfaction with results
633 (16.8)
Intolerable side effects
581 (15.4)
Weight-loss goal attained
277 (7.3)
Temporary pause/going on holiday
212 (5.6)
Inadequate GLP-1 supply
202 (5.4)
Not satisfied with the service
90 (2.4)
Unrelated health issue
58 (1.5)
Only wanted 1 order
46 (1.2)
Pregnant/trying to conceive
38 (1.0)
Migrating abroad
28 (0.7)
All other reasons (GP advice, Medication concern, Slow onboarding, etc)
81 (2.2)
Adherence – mean (SD) days
Cost
116.3 (±88)
Dissatisfaction with results
110.9 (±77.6)
Intolerable side effects
72.4 (±64.8)
Weight-loss goal attained
160.7 (±83.1)
Temporary pause/going on holiday
118.8 (±99.7)
Inadequate GLP-1 supply
188 (±85.7)
*Primary discontinuation reason data were collected from 3757 of a possible 6795 patients (consult drop-outs and patient notified MDTs).
Table 3: Discontinuation reasons and adherence.
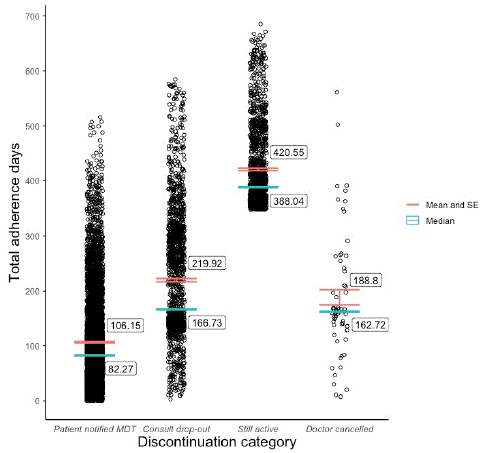
Figure 1: Adherence distribution and central tendency by discontinuation category.
A Pearson correlation test found a small, yet statistically significant positive association between age and program adherence [r(9292) = 0.038, p < 0.01]. The same test also observed a positive relationship between BMI and program adherence [r(7962) = 0.048, p < 0.01], which the investigators explored further by creating 4 BMI categories. The subsequent ANOVA [F(3,7960) = 5.9, p <0.01] and Tukey HSD post hoc tests showed a statistically significant difference in mean adherence days between the Overweight group (M=194.8, SD = 154) and the class 2 (M=179.1, SD =146 ) and 3 (M= 172, SD = 144) obese groups, and the class 1 (M =185.7, SD =151) and class 3 (M = 172, SD = 144) obese groups. Given the low representation of patients of non-Caucasian ethnicity, patients of these various backgrounds were grouped as ‘non-Caucasians’ to compare adherence with Caucasian patients. An ANOVA [F(2, 8291) = 28.49, p<0.01] and post hoc Tukey HSD test revealed a statistically significant difference between all three categories (Caucasian, non-Caucasian, Prefer not to respond), with Caucasians (M = 176.1, SD = 131) tending to adhere to the Juniper UK program for longer than non-Caucasians (M = 155.7, SD = 122).
Finally, discontinuation reasons were found to have affected adherence levels to a statistically significant degree [F(19, 4134) = 27.23, p<0.01). Patients who discontinued due to side effects (M = 72.4, SD = 64.8) tended to drop-out of the program earlier than those who considered the program too expensive (M = 116.3, SD = 88) or were not satisfied with their results (M = 110.9, SD 77.6). The highest mean scores were observed among patients who discontinued for reasons concerning migration (M=226.8, SD = 198), the inadequate supply of their desired GLP-1 RA medication (M = 188, SD = 85.7) and the attainment of the weight-loss goal (M = 160.7, SD = 83.1). A comparison of the adherence distribution and central tendency among the six most common discontinuation reasons is visualized in Figure 2.
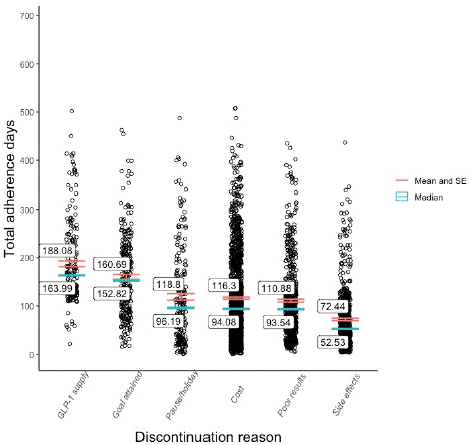
Figure 2: Adherence distribution and central tendency by discontinuation reason.
Discussion
To the knowledge of the investigators, this study is the first to generate detailed findings on patient adherence to a real-world GLP-1 RA-supported DWLS. Services of this nature hold considerable potential in the context of the obesity epidemic. Not only do they represent an opportunity for more meaningful and reliable weight-loss outcomes due to the unprecedented effect of GLP-1 RA medications observed in RCTs [15-17], but they could also feasibly increase access levels to specialist weight-loss therapy [18,19]. However, despite the proliferation of GLP-1 RA-supported DWLSs in recent years and the widespread skepticism of their quality and safety, real-world evidence on any aspect of such services has not been forthcoming. A previous study has presented data that gave an indication of adherence levels to the Juniper Australia weight-loss program [20], however these data were potentially misleading as they did not capture precise discontinuation points, but rather only demonstrated the percentage of patients who satisfied the study protocol at two follow-up points (week 14 and 32). Although the current study had its limitations, it presented a more complete picture of patient adherence to a large GLP-1 RA supported DWLS, and thus provided a series of valuable foundational insights for obesity care stakeholders and the field of digital chronic care in general.
The analysis discovered mean adherence to the Juniper UK weight-loss program among patients who commenced therapy between April 28, 2022, and April 1, 2023, was 183.1 (±148.9) days. As demonstrated by Figure 1 and the analysis’ standard deviation scores, data were widely dispersed and therefore the median adherence result of 134.8 days is arguably more informative. Nevertheless, the dispersion, it’s disaggregation into discontinuation groups (Figure 1), and the data captured on primary discontinuation reasons, illuminated the breadth of patient experiences in GLP-1 RA DWLSs. Firstly, the relatively high percentage of patients who are still active (17.3%) after nearly a year since the cohort window close date suggests the program can be sustained for a long period. Secondly, the distribution of discontinuation reasons highlighted the combination of factors that determine patient adherence, some of which are well within a DWLS’s control such as patient outcomes, service satisfaction, and side effect management; and others over which they have limited control, such as GLP-1 RA supply, patient migration or holidays, and to a certain extent, program cost. And finally, the variation in mean adherence across the discontinuation reason sub-groups suggested some interesting trends that could inform DWLS retention strategies, such as key periods for managing patient side effects, expectations around outcomes, and financial difficulties.
Conclusion
This study aimed to examine why and at what stage of the care journey patients tend to discontinue their subscription to the GLP-1 RA-supported Juniper UK weight-loss program – a DWLS that has provided care for over 15,500 British people with overweight or obesity since 2022. The analysis recorded a mean adherence period of 183.1 (±148.9) days from its cohort of 8294 patients. Program cost (38.7%) was the most common reason for discontinuation, followed by dissatisfaction with results (16.8%), intolerable side effects (15.4%), attainment of weight-loss goal (7.3%) and temporary pause/going on holidays (5.6%). Patients who were older, Caucasian and Overweight tended to adhere to the program for a statistically significantly longer period than their younger, non-Caucasian and higher BMI-class peers.
Limitations
The study had several limitations. Firstly, the sample’s relative gender and ethnic homogeneity is not representative of British society. Secondly, reasons for discontinuation were not provided by several hundred possible respondents, rendering the discontinuation reason data slightly less accurate than it may have been with a complete set of responses. Thirdly, patient BMI data was self-reported, resulting in the omission of 330 BMI entries, which were deemed erroneous (over 60). And finally, discontinuation dates for the sub-group of consult drop-out patients were estimated at 50 days from their last consult attended. Although this estimation logic was based on previously observed internal data patterns and the sound rationale of a slightly extended dosing schedule, it is ultimately an estimation and not a precise discontinuation measurement.
Author Statements
Acknowledgements
L.T conceptualized and designed the study, conducted all analyses, and drafted and revised the manuscript. M.V conducted an independent analysis, and reviewed and revised the manuscript.
Data Availability
All study data will be available from the corresponding author on written request.
Author Disclosure Statement
L.T and M.V are paid employees at Eucalyptus.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Baker C. Obesity statistics. House of Commons Library. 2023.
- Pi-Sunyer X, Astrup A, Fujioka K. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med. 2015; 373: 11–22.
- Wilding J, Batterham R, Calanna S. Once-weekly semaglutide in adults with overweight or obesity. N Eng J Med. 2021; 384: 989–1002.
- Jastreboff A, Aronne L, Ahmad N. Tirzepatide once-weekly for the treatment of obesity. N Engl J Med. 2022; 387: 205–16.
- Irvin L, Madden L, Marhsall P. Digital health solutions for weight loss and obesity: A narrative review. Nutrients. 2023; 15: 1858.
- Hinchcliffe N, Capehorn M, Bewick M. The potential role of digital health in obesity care. Adv Ther. 2022; 39: 4397–412.
- Golovaty I, Hagan S. Direct-to-consumer platforms for new antiobesity medications – concerns and potential opportunities. N Engl J Med. 2024; 390: 677-680.
- Health service delivery framework for prevention and management of Obesity. Geneva: World Health Organization. 2023.
- Evidence standards framework for digital health technologies. National Institute for Health and Care Excellence. 2022.
- Lemstra M, Bird Y, Nwanko C. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016; 10: 1547–59.
- Golovaty I, Hagan S. Direct-to-consumer platforms for new antiobesity medications – concerns and potential opportunities. N Engl J Med. 2024; 390: 677-680.
- Patel M, Wakayama L, Bennett G. Self-monitoring via digital health in weight-loss interventions: A systematic review among adults with overweight or obesity. Obesity. 2021; 29: 478–99.
- Talay L, Alvi O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism. 2024.
- Talay L, Alvi O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism. 2024.
- Pi-Sunyer X, Astrup A, Fujioka K. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med. 2015; 373: 11–22.
- Wilding J, Batterham R, Calanna S. Once-weekly semaglutide in adults with overweight or obesity. N Eng J Med. 2021; 384: 989–1002.
- Jastreboff A, Aronne L, Ahmad N. Tirzepatide once-weekly for the treatment of obesity. N Engl J Med. 2022; 387: 205–16.
- Hinchcliffe N, Capehorn M, Bewick M. The potential role of digital health in obesity care. Adv Ther. 2022; 39: 4397–412.
- Golovaty I, Hagan S. Direct-to-consumer platforms for new antiobesity medications – concerns and potential opportunities. N Engl J Med. 2024; 390: 677-680.
- Talay L, Alvi O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism. 2024.
