
Case Report
Austin Crit Care J. 2014;1(1): 3.
Fulminant Aspergillus-Related Cavernous Sinus Thrombosis in an Immunocompetent Intensive Care Unit Patient
Srinivas Rajagopala1*, Sujatha Chandrasekharan2, Sathish Kumar3 and Mohammed Rela4
1Department of Medicine, Jawaharlal Nehru Institute of Postgraduate Medical Education & Research, India
2Department of Microbiology & Serology, Jawaharlal Nehru Institute of Postgraduate Medical Education & Research, India
3Department of Neurology, Jawaharlal Nehru Institute of Postgraduate Medical Education & Research, India
4Department of Hepatobiliary surgery and Liver Transplantation, Global Hospitals and Health City, India
*Corresponding author: Srinivas Rajagopala, Medical Intensive Care Unit, Jawaharlal Nehru Institute of Postgraduate Medical Education & Research, Dhanvantri Nagar, Puducherry, 605006, India
Received: August 12, 2014; Accepted: September 12, 2014; Published: September 15, 2014
Abstract
We describe an immunocompetent liver trauma patient who developed invasive Aspergillus pneumonia and cavernous sinus thrombosis in the intensive care unit. Invasive mold infection in new niches, including critically ill patients is increasingly being recognized; this case highlights that relative immunodeficiency related to critical illness, massive transfusion and hemorrhage may predispose patients to fulminant mold-related infection. The importance of physical findings such as pupillary asymmetry in critical care is also highlighted.
Keywords: Cavernous sinus thrombosis; Fulminant fungal sinusitis; Invasive aspergillosis; Liver laceration; Immunocompetent adult
Case Presentation
A 29-year old software engineer presented with prostration following blunt injury to the abdomen in a road traffic accident. He was evaluated in another hospital half-an hour after the accident. Examination revealed tachycardia, normal blood pressure and distended abdomen. He was resuscitated with oxygen delivered by face-mask, fluids administered by large-bore peripheral lines and four group-matched whole blood units. Contrast enhanced Computed Tomography (CT) of the abdomen showed extensive liver laceration with hemoperitioneum. CT-head was normal. He was referred to this center for further management. At the initial examination in the emergency of this hospital, the patient was drowsy but oriented (Glasgow coma score 10/15). Pupils were symmetrical, reacting 3 millimeters (mm), without focal neurological deficit. Pulse rate was 128/min, respiratory rate was 26/min, blood pressure was 80/50 and the pulse oximetry saturation was 92%. Abdominal evaluation showed a tense distended abdomen; no peritoneal signs were present. No other fractures or spine injuries were evident. Initial investigations are summarized in Table 1. Review of the CT confirmed the findings and focused screening ultrasonography was normal. Initial management included elective tracheal intubation and ventilation, transfusion to a target of 30 g/dL and emergency laparotomy. Hemostasis was secured and surgical packing of the liver was performed. (Figure1). He subsequently required repeat laparotomy, coil embolization through selective hepatic artery catheterization and activated factor VIIa for control of bleeding. Heparin-free hemodiafiltration for renal failure and ventilation were continued. Empiric piperacillin-tazobactum, pantoprazole, and enteral nutrition were started. On post-operative day 5, new onset of fever, increase in FiO2 requirements and purulent secretions in the endotracheal tube were noted. Respiratory sounds were reduced in the right side. Neurological examination initially showed a non-reactive 6 mm right pupil; the left pupil was 3 mm and reacting to light. Complete restriction of right-sided ocular movements and mild chemosis developed over the next few hours. The left eye and rest of the neurological examination, including fundoscopy, was normal. Rest of the relevant investigations on day 5 was as reported (Table 1). Plain CT-head, performed due to concerns with contrast administration with acute renal failure, did not reveal any infarction or bleeding. Sinus examination showed extensive right-sided pansinusitis with soft-tissue attenuation debris in the nares and sinuses (Figure 2). Chest radiography showed right upper lobe consolidation and collapse. Flexible bronchoscopy revealed purulent secretions with mucosal irregularity of the right upper lobe main bronchus. Bronchoscopic biopsy and lavage was performed. Emergency nasal endoscopic debridement and lavage was performed from the right maxillary, frontal and ethmoid sinuses. Bacterial gram's stain and cultures were sterile. Empiric meropenem, linezolid and liposomal amphotericin B 5 mg/Kg/day were initiated. Magnetic resonance imaging (MRI) of the head without gadolinium contrast was performed (Figure 3). Bronchoscopic biopsy and sinus fluid for fungal stain showed septate hyphae with acute angle branching (Figure 4).
Test
Value
Hemoglobin
9.8 g/dL
Total leukocyte count
23,000/ÁL
Platelet count
1,20,000/ÁL
Fibrinogen
121 (Normal 200-400) g/dL
Prothrombin time
24.6 seconds (Control 12 seconds)
International Normal Ratio
2.04 (Normal 1.00)
Serum Aspartate transaminase
1190 (Normal 0-46) U/L
Serum Alanine transaminase
430 (Normal 0-50) U/L
Serum alkaline phosphatase
630 (Normal 53-128) U/L
Total bilirubin
15.63 (Normal 0.3-1.2) mg/dL
Direct Bilirubin
12.6 (Normal 0-0.3) mg/dL
Serum albumin
2.2 (Normal 3.5-4.5) g/dL
Serum creatinine
3.6 (Normal 0.5-1.2) mg/dL
Serum Sodium
136 (Normal 134-146) mEq/dL
Serum Potassium
3.6 (Normal 3.5-5.5) mEq/dL
Blood Ammonia
92 (Normal 11-36) Ámol/L
Serum arterial lactate
6.5 (Normal <2) mmol/L
Arterial PH
7.34 (Normal 7.4▒0.04)
Ventilation: FiO2 0.6, Assist volume controlled mode, frequency 15/min, Tidal volume 480 mL, Positive end-expiratory pressure 8
PaO2/FiO2
312 (Normal 450-500)
PaCO2
34 (Normal 38-42) mm Hg
Serum bicarbonate
20 (Normal 22-26) mEq/dL
Serum Phosphate
8 (Normal 2.7-4.5) mg/dL
Serum Calcium (corrected)
9.2 (Normal 8.5 - 10.1) mg/dL
Blood culture x 2
Sterile
Urine Culture
Sterile
Abdominal drain cultures
Sterile
Bronchoscopic lavage fluid
Acinetobacter spp. sensitive to imipenem, colistin and tigecycline
Fungal smear
Septate hyphae with acute angle branching
Fungal cultures
Aspergillus niger
Acid-fast stain
Negative
Table 1: Summary of investigations on Day 5 post-laparotomy.
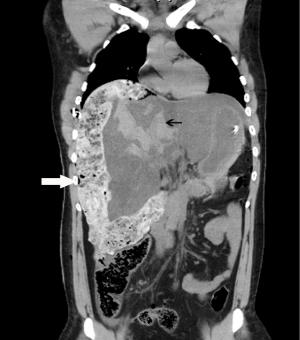
Figure 1: Reformatted coronal image of computed tomography images at
admission after control of hemostasis showing surgical packs (white arrow)
placed for control of hemorrhage from liver lacerations and hemorrhage
(black arrow) resulting in hemoperitoneum. Control of hemorrhage required
further administration of factor VIIa, angioembolisation and repeat laparotomy
at 48 hours.
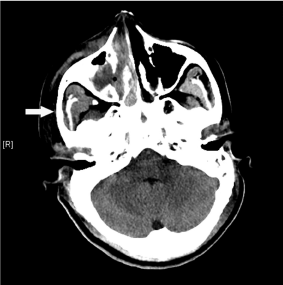
Figure 2: Plain computed tomography images of the head showing bilateral
maxillary sinusitis with plugging of the right maxillary sinusitis (white arrow)
and nasal cavity. Extensive sphenoid and ethmoid sinusitis was also present
(not shown). No evidence of bony erosion or intracranial hemorrhage or
infarction was seen.
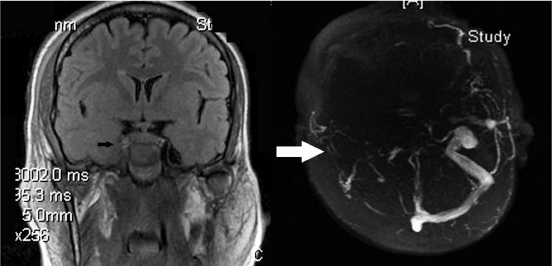
Figure 3: Coronal non-contrast Fluid attenuation inversion recovery-
Magnetic Resonance imaging (MRI, left) showing asymmetry enlargement
in the cavernous sinus with thrombosis of the internal carotid artery. MRvenography
images (right) showing a filling defect in the right-sided transverse
sinus and sigmoid dural sinus (white arrow). There is no filling in the entire
right-sided ophthalmic, inter-cavernous and petrosal dural venous sinuses.
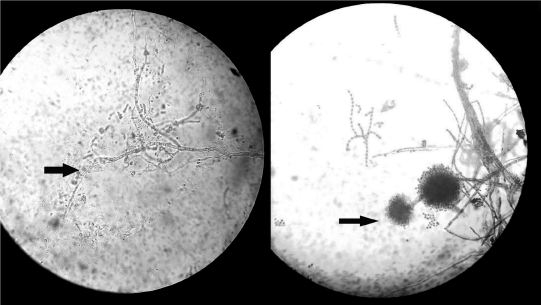
Figure 4: Photomicrographs of Potassium Hydroxide (KOH) smears
from maxillary drainage showing septate hyaline hyphae with acuteangle
branching consistent with Aspergillus species. Cultures (right) grew
Aspergillus niger.
Discussion
A diagnosis of disseminated invasive aspergillosis with acute fulminant invasive fungal sinusitis, cavernous sinus and right internal carotid artery thrombosis and right upper lobe collapse in an immunocompetent adult was made. Meropenem and liposomal amphotericin B were continued. However, the patient developed left sided hemiplegia and refractory septic shock and succumbed to his illness on day 8 of admission.
The differential diagnosis of pupil asymmetry in a ventilated patient includes raised intracranial tension, traumatic brain injury and medications. This index case highlights the importance of pupil asymmetry as a marker of third nerve palsy outside the usual context of raised intracranial tension in critical care. The cavernous sinus is a complex anatomical structure comprising a venous plexus, carotid artery, cranial nerves (third, fourth, sixth, ophthalmic and maxillary divisions of fifth), and sympathetic fibers in a very small area [1]. This small area can be affected by spread of facial (dangerous area of face) and sinus infections [bacterial and fungal], inflammatory syndromes (pseudotumors, Tolosa-Hunt syndrome), intra-sellar tumors, aneurysms of intracranial carotid artery, malignant hypertension with carotid dissection, carotid-cavernous fistula (traumatic, hypertensive) and rarely primary sinus tumors (chordomas and meningomas of sphenoid wing) [1].
Fulminant invasive fungal sinusitis results from spread of fungi from the nasal mucosa by vascular invasion into the orbit, vessels and brain parenchyma [2]. Most patients have defects in immune function (diabetes, malignancies, transplantation and chemotherapy) and disseminated invasive mold infections in immunocompetent patients are very rare [3]. A large cohort of endotracheal aspirate cultures of Intensive Care Unit (ICU) patients suggests that colonization is 100-fold as common as disease [4]. Possible factors contributing to fulminant fungal sinusitis in the index patient include the anti-inflammatory response of severe inflammatory response syndrome, massive transfusion, acute renal failure and broadspectrum antibiotics [5]. There is increasing recognition of Invasive Pulmonary Aspergillosis (IPA) in non-classical settings, especially in severe sepsis, liver failure, chronic obstructive pulmonary disease, post-operative state and TNF-α antagonist in ICU patients [6]. The incidence of IPA has been reported to range from 0.3-5.8% of ICU admissions and has an attrbutable mortality exceeding 20% [7].
MRI is better at delineating intracranial spread and gadolinium contrast-enhanced MRI can delineate spread into the orbit, paranasal sinuses, dural venous sinuses, intracranial arteries and cerebrum [8]. Diagnosis requires clinic-radiological suspicion with culture and microscopic examination of the specimens [5]. Histological specimens showing sub-mucosal fungal hyphae or tissue necrosis with minimal host inflammation suggest invasive fungal sinusitis.
Treatment of invasive fungal sinusitis requires reversal of predisposing disease, surgical debridement, and appropriate systemic antifungal. Liposomal amphotericin B at doses of 3-5 mg/kg/day is preferred in patients with renal failure (Glomerular filtration rate <50 mL/min) because the parenteral preparation of voriconazole contains sulfobutylether-β-cyclodextrin and is contraindicated in renal failure. In patients without renal failure and cerebral invasive aspergillosis, voriconazole is the preferred agent. Nasal endoscopic debridement of involved sinuses is crucial to establish the diagnosis, reduce fungal load and slow disease progression and can be performed bedside in critically ill adults. The overall mortality is around 20% but is affected by the causative organism, reversal of underlying immunosuppressive condition and time to initiation of effective therapy. Mortality is nearly universal with symptomatic intracranial extension and radical surgery should be avoided in this group.
References
- Lee JH, Lee HK, Park JK, Choi CG, Suh DC. Cavernous sinus syndrome: clinical features and differential diagnosis with MR imaging. AJR Am J Roentgenol. 2003; 181: 583-590.
- Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am. 2008; 41: 497-524.
- Tortorano AM, Dho G, Prigitano A, Breda G, Grancini A, Emmi V, et al. Invasive fungal infections in the intensive care unit: a multicentre, prospective, observational study in Italy (2006-2008). Mycoses. 2012; 55: 73-79.
- Mortensen KL, Johansen HK, Fuursted K, Knudsen JD, Gahrn-Hansen B, Jensen R, et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011; 30: 1355-1363.
- Dutkiewicz R, Hage CA. Aspergillus infections in the critically ill. Proc Am Thorac Soc. 2010; 7: 204-209.
- Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011; 49: S7-7S12.
- Trof RJ, Beishuizen A, Debets-Ossenkopp YJ, Girbes AR, Groeneveld AB. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007; 33: 1694-1703.
- Saini J, Gupta AK, Jolapara MB, Chatterjee S, Pendharkar HS, Kesavadas C, et al. Imaging findings in intracranial aspergillus infection in immunocompetent patients. World Neurosurg. 2010; 74: 661-670.