
Research Article
Austin Crit Care J. 2016; 3(1): 1015.
Efficacy and Safety of Inhaled Dry Powder Mannitol in Treating Cystic Fibrosis: A Meta-Analysis and Systematic Review of Randomized Trials
Jaeger A1, Moole V2, Dharmapuri S3, Boddireddy R4, Taneja D5,6, Ernst J7, Moole H1* and Chittivelu S5,6
1Division of General Internal Medicine, University of Illinois College of Medicine at Peoria, USA
2Division of General Internal Medicine, Mamatha Medical College, NTR University of Health Sciences, India
3Division of General Internal Medicine, Deccan Medical College, NTR University of Health Sciences, India
4Division of General Internal Medicine, Pinnamaneni Siddhartha Medical College, NTR University of Medical Sciences, India
5Division of Pulmonology and Critical Care Medicine, University of Illinois College of Medicine at Peoria, USA
6Illinois Lung and Critical Care Institute, USA
7Department of Family Medicine, University of Illinois College of Medicine at Peoria, USA
*Corresponding author: Harsha Moole, Department of Internal Medicine, University of Illinois College of Medicine Peoria, Peoria, Illinois, USA
Received: April 04, 2016; Accepted: May 23, 2016; Published: May 26, 2016
Abstract
Background: Inhaled dry powder mannitol is an osmotic agent with a potential to improve lung functions in Cystic Fibrosis (CF) patients and possibly act as a disease modifying agent.
Aim: Primary outcomes are to evaluate the improvement of lung functions.
Methods
Study selection criteria: Randomized trials that evaluated the improvement of lung functions in CF patients with the use of inhaled dry powder mannitol.
Data collection & extraction: Articles were searched in Medline, Pubmed, and Ovid journals.
Statistical method: Pooled proportions were calculated using fixed and random effects model.
Results: Initial search identified 387 reference articles, of which 31 articles were selected and reviewed. Data was extracted from 6 studies (N = 771) which met the inclusion criteria. After the treatment duration (median12 weeks), FEV1% increased by 7.23 (95% CI = 6.88 to 7.58) and 2.77 (95% CI = 2.57 to 2.97) in the pooled patients of treatment and control groups respectively. FEV1 (in ml) improved by 114.12 (95% CI = 108.96 to 119.29) and 6.80 (95% CI = 6.13 to 7.48) in treatment and control groups respectively. Odds ratio for pharyngeal pain, cough, hemoptysis and headache in treatment group compared to control group were 1.52 (95% CI = 0.91 to 2.52), 1.27 (95% CI = 0.85 to 1.90), 1.82 (95% CI = 0.97 to 3.39) and 0.80 (95% CI = 0.54 to 1.19) respectively.
Conclusion: Inhaled mannitol may be used as a chronic disease modifying treatment in patients with pulmonary CF and possibly improve the overall outcomes.
Keywords: Inhaled mannitol; Cystic fibrosis; FEV1; Outcomes; Metaanalysis and Systematic review
Introduction
Cystic Fibrosis (CF) is caused by a defective protein - Cystic Fibrosis Transmembrane conductance Regulator (CFTR), due to mutations in a gene on chromosome 7. Malfunction of CFTR protein reduces the water content in various epithelial secretions (pancreatic, biliary, respiratory), resulting in difficult to clear viscous secretions. In the respiratory tract, these viscous secretions cause chronic obstruction of airways and facilitate chronic infection with pathogenic bacteria (secondary to decreased ability to kill bacteria, progressive colonization and bacterial biofilm formation on epithelial surfaces). Inflammatory reaction due to this chronic infection results in tissue damage and bronchiectasis [1,2]. CF disease is usually characterized by progressive decline in lung function superimposed with intermittent acute exacerbations. The disease flares are usually treated with antibiotics. CFTR modulators (ivacaftor) can be used in patients with a few specific type of gene mutations evident on CFRT genotyping [3-5]. Chronic treatments include short-acting inhaled beta-2-adrenergic receptor agonists, inhaled DNase I (dornasealfa), hypertonic saline and chronic azithromycin therapy. Inhaled hypertonic saline, due to its osmotic effect, draws fluid on to the surface epithelium and reconstitutes the environment of normal respiratory airway surface. Inhaled dry powder mannitol (a sugar alcohol) is a drug that works by the same osmotic principle and draws fluid into the lumen of respiratory airways. It facilitates mucous clearance [6,7] increases ciliary beat frequency [8], acts as an expectorant by stimulating cough, clears the bacteria and debris lining the diseased respiratory epithelium. Eventually these actions translate into improved FEV1 [9-12]. Studies have shown improved FEV1/lung function [12,13], improved surface properties and hydration [14] with the use of inhaled mannitol. Two studies [13,15] have shown reduced CF exacerbation rates with the use of 400 mg mannitol by inhalation twice daily. As mentioned above, several studies have been published that evaluated the efficacy and safety profile of inhaled mannitol [12,13,15-18]. Although individual studies have shown encouraging results with the use of inhaled mannitol, Food and Druginhaled beta-2-adrenergic receptor agonists, inhaled DNase I (dornasealfa), hypertonic saline and chronic azithromycin therapy. Inhaled hypertonic saline, due to its osmotic effect, draws fluid on to the surface epithelium and reconstitutes the environment of normal respiratory airway surface. Inhaled dry powder mannitol (a sugar alcohol) is a drug that works by the same osmotic principle and draws fluid into the lumen of respiratory airways. It facilitates mucous clearance [6,7] increases ciliary beat frequency [8], acts as an expectorant by stimulating cough, clears the bacteria and debris lining the diseased respiratory epithelium. Eventually these actions translate into improved FEV1 [9-12]. Studies have shown improved FEV1/lung function [12,13], improved surface properties and hydration [14] with the use of inhaled mannitol. Two studies [13,15] have shown reduced CF exacerbation rates with the use of 400 mg mannitol by inhalation twice daily. As mentioned above, several studies have been published that evaluated the efficacy and safety profile of inhaled mannitol [12,13,15-18]. Although individual studies have shown encouraging results with the use of inhaled mannitol, Food and Drug Administration (FDA) has declined the approval of this medication due to efficacy and safety (increased hemoptysis) concerns. There is ambiguity regarding the benefits and risks of mannitol therapy. In this meta-analysis we aim to pool the evidence regarding the efficacy and safety of inhaled mannitol in cystic fibrosis patients with pulmonary manifestations.
Methods
Aim
Primary outcomes are to evaluate the improvement of lung functions (FEV1 in ml, FEV1%, FVC in ml, FVC%) in CF patients that received inhaled dry powder mannitol 400 mg twice daily as a treatment intervention and compare it to a control arm that received inhaled mannitol 50 mg twice daily. Secondary outcomes are to compare the adverse events (pharyngeal pain, cough, hemoptysis and headache), quality of life measured by Cystic Fibrosis Questionnaire- Revised (CFQ-R) [19], and reduction in CF pulmonary exacerbation in treatment group versus control group.
Study selection criteria
Inclusion criteria:
- Patients diagnosed with CF based on standard criteria [20].
- Patients should be capable of performing reproducible spirometry according to American thoracic society criteria [21].
- Treatment arm should have received Inhaled mannitol 400 mg twice daily and control arm should have received a small amount of inhaled mannitol (40-50 mg twice daily).
- Prior to each treatment, patients received pretreatment with a bronchodilator - Salbutamol 400 micro grams or terbutaline 1 mg via a Metered-Dose Inhaler (MDI) and a volume spacer.
- Patients with age above six years were included in this metaanalysis.
- Patients with predicted FEV1 of 40% to 91%.
- In Jaques et al. [12], for subjects using hypertonic saline nebs prior to the study, a two week wash out period was required to be eligible for study enrollment.
- Minasian et al. [16] included patients with age 8-18 years.
- Patients with current asthma, supplemental home oxygen requirement, colonization with Burkholderiacepacia, terminal illness, breast feeding, pregnancy, hemoptysis of more than 60ml in the last 12 months, portal hypertension, stroke or myocardial infarction in the last 3 months were excluded.
- Patients with airway hyper-responsiveness (defined as greater than 15% fall in FEV1 after an airway challenge with mannitol) were excluded. Patients with known hypersensitivity to inhaled mannitol.
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007; 132: 1631-1636.
- Puchelle E, Bajolet O, Abély M. Airway mucus in cystic fibrosis. Paediatr Respir Rev. 2002; 3: 115-119.
- United States Food and Drug Administration, MedWatch, Safety labeling changes for Kalydeco (ivacaftor) tablets. December 2014.
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009; 106: 18825-18830.
- Patel S, Sinha IP, Dwan K, Echevarria C, Schechter M, Southern KW Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database Syst Rev 2015; 3.
- King M, Rubin BK. Pharmacological approaches to discovery and development of new mucolytic agents. Adv Drug Deliv Rev. 2002; 54: 1475-1490.
- Daviskas E, Anderson SD. Hyperosmolar agents and clearance of mucus in the diseased airway. J Aerosol Med. 2006; 19: 100-109.
- Brannan JD, Gulliksson M, Anderson SD, Chew N, Kumlin M. Evidence of mast cell activation and leukotriene release after mannitol inhalation. EurRespir J 2003; 22: 491-496.
- Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J. 1999; 14: 678-685.
- Daviskas E, Anderson SD, Eberl S, Chan HK, Young IH. The 24-h effect of mannitol on the clearance of mucus in patients with bronchiectasis. Chest. 2001; 119: 414-421.
- Wills PJ, Cole PJ. Mucolytic and mucokinetic therapy. Pulm Pharmacol. 1996; 9: 197-204.
- Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest. 2008; 133: 1388-1396.
- Bilton D, Robinson P, Cooper P, Gallagher CG, Kolbe J, Fox H, et al. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J. 2011; 38: 1071-1080.
- Daviskas E, Anderson SD, Jaques A, Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest. 2010; 137: 861-868.
- Aitken ML, Bellon G, De Boeck K, Flume PA, Fox HG, Geller DE, et al. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med. 2012; 185: 645-652.
- Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax. 2010; 65: 51-56.
- Teper A, Jaques A, Charlton B. Inhaled mannitol in patients with cystic fibrosis: A randomised open-label dose response trial. J Cyst Fibros. 2011; 10: 1-8.
- Middleton A, Robinson PD, McKay K, Jaffe A, Selvadurai H. A pilot study of inhaled dry-powder mannitol during cystic fibrosis-related pulmonary exacerbation. Eur Respir J. 2015; 45: 541-544.
- Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005; 128: 2347-2354.
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998; 132: 589-595.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995; 152: 1107-1136.
- Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992; 304: 1491-1494.
- Jadad AR, Moore RA, Carroll D. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clin Trials. 1996; 17: 1-12.
- Stroup DF, Berlin JA, Morton SC. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008-2012.
- Stuart A, Ord JK. Kendall's Advanced Theory of Statistics. 6th Edn. London: Edward Arnold; 1994.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7: 177-188.
- Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. In Egger M, Smith GD, Altman DG, (Edn). Systematic Reviews in Health Care. Meta-analysis in context. London: BMJ Books. 2001.
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006; 25: 3443-3457.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50: 1088-1101.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001; 323: 101-105.
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001; 54: 1046-1055.
- Brannan JD, Anderson SD, Perry CP. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4.5%) saline. Respir Res 2005; 6:144.
- Proesmans M, Vermeulen F, De Boeck K. What's new in cystic fibrosis? From treating symptoms to correction of the basic defect. Eur J Pediatr. 2008; 167: 839-849.
- Fuchs HJ, Borowitz DS, Christiansen DH. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994; 331: 637-642.
Exclusion criteria:
Data collection & extraction
Articles were searched in Medline, PubMed, Ovid journals, EMABSE, Cumulative Index for Nursing & Allied Health Literature, ACP journal club, DARE, International Pharmaceutical Abstracts, old Medline, Medline nonindexed citations, OVID Healthstar, and Cochrane Central Register of Controlled Trials (CENTRAL). The search was performed for the years 1966 to January 2016. Abstracts were manually searched in the major pulmonology and critical care medicine, internal medicine journals for the past 3 years. Study authors for the abstracts included in this analysis were contacted when the required data for the outcome measures could not be determined from the publications. The search terms used were inhaled mannitol, cystic fibrosis, FEV1, lung function, meta-analysis, systematic review, outcomes, and complications. Two authors (AJ and VM) independently searched and extracted the data into an abstraction form. Any differences were resolved by mutual agreement. The agreement between reviewers for the collected data was quantified using the Cohen’s κ [22].
Definition
Clinical stability was defined as absence of antibiotic treatment in the two weeks prior to study enrollment and absence of systemic illnesses of any kind in the week prior to study entry. Active asthma is defined as “patients with ongoing signs and symptoms of asthma”. CF pulmonary exacerbation was defined based on Fuch’s criteria 4.
Quality of studies
Clinical trials designed with a control and treatment arms can be assessed for quality of the study. A number of criteria have been used to assess this quality of a study (e.g. randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome) [23,24]. There is no consensus on how to assess studies designed without a control arm. Hence, these criteria do not apply to studies without a control arm [24].
Statistical methods
This meta-analysis was performed by calculating pooled proportions. First the individual study proportion of FEV1 increase from baseline, FVC improvement, adverse events etc, was transformed into a quantity using Freeman-Tukey variant of the arcsine square root transformed proportion. The pooled proportion is calculated as the back-transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian-Laird weights for the random effects model [25,26]. Forest plots were drawn to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the Forest plots indicates the assigned weight to that study. The heterogeneity among studies was tested using Cochran’s Q test based upon inverse variance weights [27]. If p value is > 0.10, it rejects the null hypothesis that the studies are heterogeneous. The effect of publication and selection bias on the summary estimates was tested by both Harbord-Egger bias indicator [28] and Begg-Mazumdar bias indicator [29]. Also, funnel plots were constructed to evaluate potential publication bias [30,31]. Microsoft Excel 2013 software was used to perform statistics for this meta-analysis.
Results
Initial search identified 387 reference articles, in which 31 articles were selected and reviewed. Data was extracted from 6 studies [12,13,15-18] (N=771) that evaluated the efficacy and safety of inhaled mannitol in CF lung disease patients, which met the inclusion criterion. All the studies are published as full text articles. Figure 1 shows the flow diagram of search results. All the pooled estimates given are estimates calculated by the fixed effect model. Fixed effect model was preferred to random effects model for better accuracy based on the nature of individual study characteristics and heterogeneity. All the six studies included in this meta-analysis were prospective trials. Three trials [12,16,17] were cross over prospective studies. Three trials [12,16,18] were single centered studies. The total number of patients included in this meta-analysis is 771, with amale population (47%). Median age of the patients was 20 years. Patients were treated with inhaled mannitol 400mg twice daily for a range of twelve days to 52 weeks, with a median of twelve weeks. Table 1 shows the baseline characteristics of the studies. The p for chi-squared heterogeneity for all the pooled accuracy estimates was > 0.10.The agreement between reviewers for the collected data gave a Cohen’s κ value of 1.0.
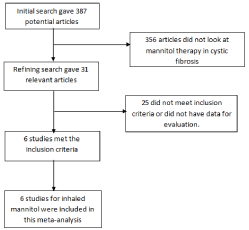
Figure 1: Flow diagram: Search results.
Sl. No
Study
Country
Type
N
N- M
Rx Time
FEV1% Increase#
1
Jaques et al, 2008
Australia
RCO
39
21
2 weeks
7
2
Minasian et al, 2010
UK
RCO
20
20
12 weeks
6.7
3
Teper et al, 2011
Argentina, Australia
RCO
48
48
13 weeks
8.75
4
Bilton et al, 2011
Multi@
RCT
324
192
52 weeks
6.5
5
Aitken et al, 2012
Multi.
RCT
318
192
52 weeks
8.22
6
Middleton et al, 2015
Australia
RCT
22
11
12 days
7.1
RCT – Randomized Controlled Trial; RCO – Randomized Cross Over study.
N – Total number of patients in each study; N-M – Total number of patients in inhaled mannitol treatment wing; Rx time – Treatment duration in each study.
#FEV1 % increase in treatment group; @ UK, Australia, Ireland, New Zealand; $ USA, France, Belgium, Australia, The Netherlands, Germany.
Table 1: Basic characteristics of the included studies.
Efficacy of inhaled mannitol
After the treatment interventions, FEV1% increased by 7.23 (95% CI = 6.88 to 7.58) and 2.77 (95% CI = 2.57 to 2.97) in the pooled patients of treatment and control groups respectively. FEV1 (in ml) improved by 114.12 (95% CI = 108.96 to 119.29) and 6.80 (95% CI = 6.13 to 7.48) in pooled patients of treatment and control groups respectively. In the pooled patients of treatment group, FVC (in ml) improved by 136.14 (95% CI = 129.84 to 142.45) and FVC% increased by 5.43 (95% CI = 5.15 to 5.72) after treatment. Heterogeneity among the individual studies was assessed with I2 (inconsistency) = 79.2% (95% CI = 42.9% to 88.8%). Bias indicator was Egger: bias = 0.53 (95% CI = -5.05 to 6.12) P = 0.80. Figure 2 is a forest plot representing the pooled and individual rates of improvement in FEV1% in treatment group. Figure 3 is a funnel plot assessing the publication bias for same variable.
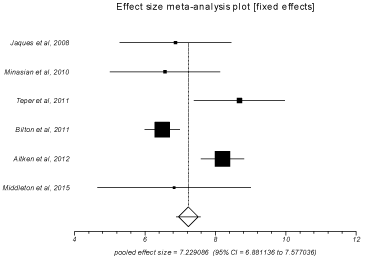
Figure 2: Forest plot - Individual study proportions and the pooled estimate
for patients with improvement in FEV1% in treatment group (Fixed effects).
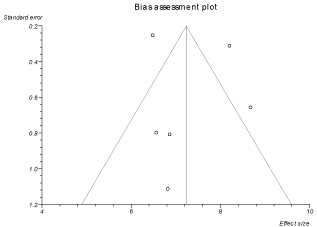
Figure 3: Funnel plot assessing for publication bias (for improvement in
FEV1% in treatment group).
Morbidity associated with inhaled Mannitol
In the pooled proportion of patients, the odds ratio for pharyngeal pain, cough, hemoptysis and headache in treatment group compared to control group were 1.52 (95% CI = 0.91 to 2.52), 1.27 (95% CI = 0.85 to 1.90), 1.82 (95% CI = 0.97 to 3.39) and 0.80 (95% CI = 0.54 to 1.19) respectively. Due to the limited data available from individual studies, we were not able to pool the results for quality of life, CF pulmonary exacerbations, intravenous antibiotics and hospitalizations. Mannitol therapy was not associated mortality. Heterogeneity among the individual studies was assessed with I2 (inconsistency) = 84.6% (95% CI = 58.8% to 91.6%). Bias indicator is Egger: bias = 2.18 (95% CI = -4.96 to 9.33) P = 0.40. Figure 4 is a forest plot representing the odds ratio for hemoptysis in treatment group vs. placebo group. Figure 5 is a funnel plot assessing the publication bias for same variable.
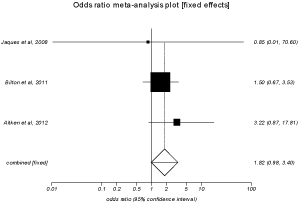
Figure 4: Forest plot - Individual study proportions and the pooled estimate
for odds ratio of hemoptysisin treatment group vs. placebo group (fixed
effects).
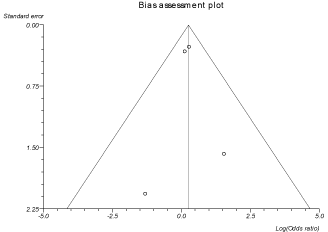
Figure 5: Funnel plot assessingfor publication bias (odds ratio for hemoptysis
in treatment group vs. placebo group).
Discussion
In patients with pulmonary manifestations of CF, there is an active search for novel agents to help reduce the disease burden. Natural progression of the disease involves progressive decline in lung function due to the altered physiology (thick mucus in respiratory epithelium, airway obstruction, bacterial colonization, inflammatory reaction, and destruction of airways) that is further deteriorated by acute bacterial pulmonary exacerbations. Facilitating the clearance of thick mucus in the airways, there by clearing the bacterial colonization would help slow the progression of the lung function decline. Currently DNase I (mucolytic agent) and hypertonic saline (osmotic agent) are the standard of care treatments to reconstitute the normal airway surface environment. Inhaled mannitol has shown promising results as an osmotic agent that improves lung function. There are six studies done evaluating the safety and efficacy inhaled mannitol in CF pulmonary disease. In order to assess the clinical benefit of a therapeutic intervention in patients with CF, currently FEV1 is the most established objective measurement, this however may not co-relate with clinical outcomes related to mortality and morbidity. Jaques et al. [12] was a randomized, placebo-controlled, crossover study performed over a two week period, in 39 patients with mild to moderate CF lung disease. Treatment group received 420 mg of inhaled mannitol twice daily and the placebo group received 30 mg inhaled mannitol twice daily. Absolute FEV1 improvement and FEV1% increase from baseline in mannitol group were 121 mL (95% CI, 56.3 to 185.7) and 7.0% (95% CI, 3.3 to 10.7) respectively, compared to 0 mL (95% CI, -64.7 to 64.7) and 0.3% (95% CI, -3.4 to 4.0; p < 0.001) respectively in placebo group. Mannitol therapy was not associate with any serious adverse effects. Quality of life seemed to improve in inhaled mannitol group, especially in the respiratory domain measured by CFQ-R. Mean change in CFQ-R scores in respiratory domain in treatment and placebo group were 4.7 (95% CI, -1.8 to 11.2) and 0.7 (95% CI, -7.2 to 5.8). Minasian et al. [16] was a randomized cross over study performed over twelve weeks, where 20 patients completed the study. In the inhaled mannitol group (400 mg twice daily), mean increase in FEV1 was 110 ml (6.75%), p=0.055 above baseline. Their results demonstrated that inhaled mannitol was as effective as rhDNase in improving lung function, and a combination of inhaled mannitol plus rhDNase did not show improved outcomes. Six patients withdrew from the study reporting cough as the main reason. Inhaled mannitol is a bronchoconstrictor, especially in patients with reactive airway disease [32]. Hence, patient getting inhaled mannitol should be pre-medicated with a short acting bronchodilator. Mannitol also increases the fluid content in the respiratory airways, mobilizing the thick mucus and debris, which could act as a noxious stimuli, stimulating the cough receptors. Due to these inherent properties of mannitol, cough is an anticipated therapeutic effect of mannitol. Among all the studies included in this meta-analysis, it is important to note that cough was not troublesome enough in most of the patients that prompted them to withdraw from the study. Results of our meta-analysis gave a pooled odds ratio of 1.27 (95% CI = 0.85 to 1.90) for the incidence of cough as an adverse event in treatment group versus control group. Teper et al. [17] was a randomized, cross over, dose response study performed over two weeks in 48 patients, comparing the lung function parameters after treatment with various doses of inhaled mannitol (twice daily doses of 400 mg vs. 240 mg vs. 120 mg vs. 40mg). Highest FEV1 improvement (8.75% increase from baseline) was noted with 400 mg twice daily dose. Mean change in respiratory domain of CFQ-R with 400 mg mannitol was 13.5 compared to 1.15 in 40 mg mannitol. Serious adverse effects were not noted in the 400 mg group. Bilton et al. [13] was a randomized study performed in 324 CF patients. FEV1 increased by 119 ml (6.5%) in treatment group (400 mg twice daily) compared to 26 ml (2.4%) in control group (50 mg twice daily) after 26week treatment period. These results were maintained at 52 weeks treatment period. Mannitol group has 35.4% reduction in CF exacerbations compared to control group. Hemoptysis was reported either as an exacerbation of CF exacerbation (15.8% vs. 15.3%) or an adverse event (11.9% vs. 8.5%) in treatment and control groups respectively. Hemoptysis is an expected complication of CF lung disease and hence it is not surprising to notice hemoptysis in this patient population. Among the studies included in this meta-analysis, hemoptysis (either occurring as an adverse event or as a naturally occurring event in CF lung disease or exacerbation) incidence was either nil or almost equal in treatment and control groups. Pooled odds ratio for hemoptysis in treatment group versus control group was 1.82 (95% CI = 0.97 to 3.39). Aitken et al. [15] was a randomized study, blinded for 26 weeks followed by 26 weeks open label. Treatment group (inhaled mannitol 400 mg twice daily) showed FEV1 improvement of 105 ml (8.2 % above baseline) with acceptable safety profile. Fewer exacerbations (Hazards ratio 0.74%, 95% CI 0.42 to 1.32, p=0.31) were noted in treatment group compared to control group (inhaled mannitol 50 mg twice daily). Middleton et al. [18] was a randomized trial on patients is CF pulmonary exacerbation. Treatment group and control group received inhaled mannitol 400 mg and 50 mg respectively. Patients received mannitol therapy for 12 days, as an add-on to standard therapy for CF pulmonary exacerbation. Mean difference for FEV1% at follow up (treatment group minus control group, adjusted for baseline FEV1) was 5.4. They reported that inhaled mannitol could be feasible in inpatient setting. There are no establishes guidelines to recommend the use of a therapeutic agent based on FEV1 improvement. In CF patients, the widely used mucolytic agent rhDNase showed an FEV1 improvement of 5.8% at 24weeks of treatment [33,34]. Hypertonic saline (4 ml of 7% saline twice daily) is a commonly used osmotic agent that showed 68 ml improvement in FEVI, 56% reduction in CF exacerbations. In our meta-analysis, FEV1 increased by 114 ml or 7.23% in the pooled patient population, which could definitely be considered as a significant improvement when compared to the currently available mucolytics and osmotic agents.
Strengths of this meta-analysis include the high quality methodology of statistical analysis, high quality methodology used in individual studies, relatively high number of studies that met the inclusion criteria, and total number of patients included in this analysis (N = 771). All the studies included in this analysis were prospective studies with randomization, which inherently is a superior study design.
Limitations of this meta-analysis are: Due to the nature of data available from individual studies, we were not able to pool the evidence for inhaled mannitol on quality of life, reduction in CF pulmonary exacerbations, intravenous antibiotic usage and hospitalizations. Three studies [12,16,17] included in this meta-analysis were cross over studies. The disadvantages associated with these studies are potential carry over effects and medication interactions. These studies have used a two week wash out period to mitigate these potential design flaws.
Studies with statistically significant positive results tend to be published and cited. Additionally, smaller studies may show larger treatment effects compared to larger studies. This publication and selection bias may affect the summary estimates. The bias can be estimated using Egger bias indicators and the construction of funnel plots, whose shape can be affected by bias. In the present meta-analysis and systematic review, bias calculations both Egger [28] and Begg- Mazumdar [29] bias indicators showed no statistically significant bias. Furthermore, analysis using funnel plots showed no significant publication bias among the studies included in the present analysis.
Conclusion
In CF patients with lung manifestations, use of 400 mg inhaled mannitol twice daily has improved the lung function parameters over six month treatment periods. Side effect profile of treatment and control group were comparable. Hence, inhaled mannitol has the potential to be used as a standard of care chronic disease modifying agent in patients with pulmonary CF and possibly improve the overall outcomes.
References