
Research Article
Austin Crit Care J. 2016; 3(1): 1016.
Thrombocytopenia in Septic Patients - An Observational Cohort Study in Medical Intensive Care Unit
Lakshman R1*, Purushothaman SS2, Balakrishnan S2 and Nair SG2
1Fortis Memorial Research Institiute, Gurgaon, Inida
2Department of Anaesthesiology & Critical Care, Amrita Institute of Medical Sciences, Kochi, India
*Corresponding author: Ramachandran Lakshman, 112, Ashoka Enclave, Sector-34/35, Faridabad, Haryana, India
Received: September 16, 2015; Accepted: May 30, 2016; Published: June 01, 2016
Abstract
Context: Correlation of platelet count with mortality in patients with sepsis.
Introduction: Early detection of sepsis in critically ill patients and suitable intervention is known to decrease mortality. Fall in platelet count has been seen to occur early and is associated with poorer prognosis in previous studies in surgical and trauma patients in Intensive Care Units (ICU). The aim of this study was to evaluate the incidence, risk factors and outcome in septic patients who developed thrombocytopenia in a medical ICU. The time course of change in platelet counts in these patients was also compared with the established prognostic markers like APACHE IV in predicting mortality.
Design: 30-bedded MICU in a tertiary care center
Methodology: All septic patients >12 years admitted in the ICU with duration of stay > 48 hours were included in the study. The platelet counts were daily recorded along with severity scores like APACHE IV. The primary end point was 28-day mortality. Receiver-Operator Characteristic (ROC) curve and Kaplan-Meier survival analysis was done to determine the cutoff level and the survival probability of the patients with a falling platelet count.
Results: 104 septic patients were studied. Sixty three (60%) of patients developed thrombocytopenia out of which 35 (33%) patients were thrombocytopenic at admission. Time to reach the nadir platelet value was 5.3 days. No risk factors were found to be associated with thrombocytopenia. Nadir thrombocytopenia, instead of admission thrombocytopenia was found to be a better marker for predicting mortality p=0.016. Nadir thrombocytopenia had a higher discriminative value for mortality prediction than admission APACHE IV score on ROC curve. A platelet count drop by >30% was found to be associated with higher mortality.s
Conclusion: Thrombocytopenia is frequent in critically ill medical patients. No risk factors were responsible for its development. Nadir thrombocytopenia and decline in platelet count by >30% has a significant predictive value for 28- day mortality.
Keywords: Sepsis, Thrombocytopenia, Prognosis, 28-day mortality, APACHE IV
Introduction
Development of sepsis in patients admitted in the Intensive Care Unit (ICU) is associated with a mortality of 20-30% which may go up to 50-60% if associated with septic shock [1,2]. Early detection and intervention (golden hour) in sepsis has improved survival considerably [3]. Many biomarkers such as C-Reactive Protein (CRP), Procalcitonin (PCT), Total Leucocyte Count (TLC), Triggering Receptor Expressed On Myeloid Cell1 (TREM-1), serum albumin levels, etc, have been investigated for early diagnosis and prognosis in sepsis [4]. On the other hand, biological variables like Central Venous Oxygen Saturation (ScVO2) and serum lactate have already been included in the accepted management protocol of sepsis as biomarkers for its early detection [5]. Platelet count is another acute phase reactant under investigation. Measurement of platelet count being cost effective, easily available, can be done serially and may be a useful biomarker, especially in resource constrained setups.
Platelets have a pivotal role in haemostasis and thrombus formation as well as in host defence and hence, a reduction in platelet count may potentially influence the outcome of the critically ill patients [6]. Thrombocytopenia has already been shown to be associated with worsening prognosis in earlier studies on surgical and trauma patients [7].
Thrombocytopenia is a common occurrence in critically ill patients. Various mechanisms like increased platelet destruction, hemodilution, decreased marrow production and increased splenic sequestration are postulated for the development of thrombocytopenia in ICU [6,7]. Sepsis has been found to be most commonly associated with thrombocytopenia [8,9] On the other hand, thrombocytopenia has also been shown to be an independent prognostic marker and thus complimentary to establish markers like APACHE, SOFA, SAPS, etc., in patients of sepsis, septic shock or merely with evidence of new onset blood stream infection in ICU [10].
The advantage of using platelets as a predictor of mortality in critically ill patients is the dynamic nature of the daily platelet count, which takes the disease progression into account in contrast to various mortality scores which use only the worst parameters within the first 24 hr after admission or at admission [11]. Various studies on the adult patients have shown that it is not the absolute level of platelets but the change (drop) in the platelet levels that are more suggestive of poorer prognosis in terms of mortality and longer ICU stay [11,21]. Absolute platelet count and a fall in platelet count as prognostic and diagnostic markers of sepsis has not been evaluated frequently in many Indian ICUs. Agrawal et al. studied the variations in platelet count with the mortality in paediatric ICU. They concluded that a drop in platelet count and absolute thrombocytopenia were independently related to mortality [24]. However, similar data is lacking in Indian scenario, where being a cost effective diagnostic modality it may be a promising marker. Hence, to study the trend of platelet count in patients of sepsis and comparing it with the present established markers of severity of diseases might be useful as platelet count estimation is a simple, easily available and relatively cheap investigation. Our primary objective was to study the incidence of thrombocytopenia and its prognostic value in predicting mortality and Length of Stay (LOS) in critically ill septic patients. The secondary objective was to study the time trend of platelet count and its association with mortality and LOS. We also compared the efficacy of thrombocytopenia in predicting the above outcomes vis-à-vis other present prognostic markers like CRP, ScVO2, serum lactate, APACHE IV score.
Subjects and Methods
The study was a prospective, observational, cohort study and was approved by the ethical committee of the hospital. We enrolled all patients admitted from August 2010 till August 2011 in a 30 bedded medical. Sepsis was defined as per the current guidelines [12]. All patients who presented with sepsis on admission or developed new sepsis during their stay in ICU were included. Patients excluded were those with history of haematological malignancies, past use of chemotherapeutic drugs, platelet disorders, patients with mechanical cardiac valves, patients with splenectomy or hypersplenism, alcohol abuse, non-septic causes of SIRS like trauma, surgery, burns and immune-complex mediated diseases like dengue and SLE. All patients who died within 48 hours of inclusion were also excluded from the study. The patients were followed from the day of admission in MICU to their discharge, death or continued stay in ICU.
Platelet counts were determined daily throughout the ICU stay by automated cell counter. Thrombocytopenia was defined as a platelet count < 150 ×109/L. Patients with thrombocytopenia were further subdivided into mild (101-149×109/L), moderate (51-100 ×109/L), severe (21-50×109/L) and very severe (= 20 ×109/L), as in the recent Sepsis-Related Organ Failure Assessment score study [13,14]. Nadir platelet count was defined as the lowest platelet count recorded during the ICU stay. Percentage drop in the platelet count was taken as a percentage of the difference between the admission platelet count and the nadir platelet count for each patient. ICU acquired thrombocytopenia was considered when the patient was admitted with normal platelet count which dipped below 150×109/L subsequently during ICU stay. All patients received standard ICU care as per our protocols for antibiotics, mechanical ventilation, nutrition, blood and blood product transfusion and inotropic support. Data collected included age, sex, source of sepsis, use of medications in the past and during the present stay in the ICU which can affect the platelet count like heparin, NSAIDS, furosemide, β-lactams and antiplatelet drugs, use of mechanical ventilation and requirement of inotropic supports. Prognostic markers of sepsis like ScVO2 and serum lactate levels were also noted within 24 hours of the ICU admission or at the onset of the sepsis.
Acute Physiological and Chronic Health Evaluation IV (Apache IV) scoring system was used to estimate the severity of the disease or the number of organ failures within 24 hours of the admission [15]. Statistical analysis was performed using statistical software SPSS 14. Results are expressed as numerical values (percentage) for the categorical variables, and median (Interquartile Range [IQR] for continuous variables). Continuous variables were compared with student’s T test for normally distributed variables and mannwhitney test was used for non-parametrically distributed variables. The chi-square test and fisher’s exact test were used to compare the categorical variables. Receiver-Operator Characteristic (ROC) curve analysis was done to see the prediction of mortality by platelet count and to determine the cut-off level for platelet count for determining increased mortality. We also tried to determine the percentage decline of the platelet count from the admission which predicts the increased mortality again by using the ROC curve. After categorizing the platelet decline into four categories (<10%, 10-30%, 30-60 %, > 60%) we used the kaplan-meier survival analysis to determine the survival probability of the patients with falling platelet count. All p-values were two-sided and were considered significant at < 0.05.
Results
762 patients were admitted to our ICU over a period of one year. Of these, 467 patients were diagnosed as having sepsis. 319 patients did not fit into the inclusion criteria and thus were excluded. 148 patients formed the study group. Of these, 43 died within 48 hours of inclusion. Hence, the final data analysis was done for 105 patients (Figure 1). The Mean age of the study group was 56.4 years (range=17-84). Major sources of sepsis as well as the characteristic distribution of the study patients are demonstrated in Table 1.
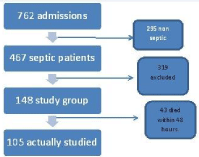
Figure 1: Flow of patients through the trial.
Characteristics
Mean
Ci 95%
Age in years
56.4 (53.3-59.4)
±3.05
APACHE IV
61.42 (58-64.8)
±3.44
Platelet count on admission
227.55 (200-255)
±27.53
Nadir platelet count
140.15 (118.8-161.4)
±21.38
Time taken to reach nadir platelet count
5.32 (4.4-6.22)
±0.96
CRP in mg/L
114.62 (98.9-130.2)
±15.62
SCVO2 in %
76.79 (74.0-79.5)
±2.77
Lactate levels in mmol/L
2.718 (2.2-3.1)
±0.42
Length of stay in days.
10.13 (11.4-8.8)
±1.27
Characteristics
Number
Percentage (%)
Male
70
66.7
Female
35
33.3
Source Of Sepsis
Urosepsis
27
25.7
Pneumonia
35
33.3
CNS infection
5
4.8
Intraabdominal infection
13
12.4
BSI
8
7.6
unknown
27
16.2
Severity Of Thrombocytopenia In Study Group
Mild((101-149× 109/L)
11
10.5
Moderate(51-100× 109/L),
16
15.2
Severe(50-20× 109/L),
5
4.8
Profound(<20× 109/L)
3
2.9
Patients on Mechanical ventilation (MV)
64
61.0
Patients on Ionotropes
53
50.5
28 day mortality
31
29.5
Table 1: Demographic and Icu Parameters (N=105).
Thrombocytopenia was present in 35 (33.3%) patients on admission. But the numbers increased to 63 (60%) during their stay in ICU. On an average, 5.32 days were taken by the platelets to reach their lowest value (nadir) during their stay. The distribution of patients into various grades of severity of thrombocytopenia is shown in Table 1, Table 2 and Table 3 shows the comparison of clinical data in patients with and without thrombocytopenia (both at admission and at their nadir). Patients with thrombocytopenia had higher APACHE IV scores than those patients who never developed thrombocytopenia during their stay (Figure 2). Thrombocytopenia on admission (n=35) was not associated with significantly increased mortality (p=0.749). However, there was a significant association with mortality in overall patients with thrombocytopenia (admission plus newly developed) (n=63) (p=0.016). Patients with nadir thrombocytopenia?had higher lactate levels (p=0.009) and longer periods of stay in ICU (p=0.039). Lower the nadir platelet count, higher was the mortality rates, as indicated by the increasing death rates, according to the severity: 10% for mild; 37.03% for moderate; 52.63% for severe and 57.14% for profound thrombocytopenia. There was no correlation seen among thrombocytopenic patients (admission as well as new onset) with their age, sex, length of stay and other prognostic markers like, CRP levels and ScVO2 levels. Various risk factors for the development of thrombocytopenia were analyzed as shown in Table 4. In our study factors like presence of blood stream infection, use of mechanical ventilation, use of drugs including inotropes, heparin, beta lactam antibiotics, non-steroidal anti inflammatory drugs, antiplatelet drugs, furosemide and associated pre-existing comorbidities were not shown to be independent risk factors contributing to the development of thrombocytopenia in ICU. The mortality rate in our study was 29.5% (31 out of 105). Of these 31 patients, 25 had thrombocytopenia at the time of death. Out of these 25 patients, 12 had thrombocytopenia on admission while 13 developed new onset thrombocytopenia (ICU acquired). Sepsis severity score like APACHE IV and other prognostic markers like ScvO2 and lactate levels (on admission day) were analyzed (Table 5) to look for their association with higher mortality. Higher lactate levels (survivors=2.473, non survivors=3.29) were associated with higher mortality (p=0.007). APACHE IV score was found to be higher in the non-survivor group (survivor=60, non-survivor=65) but it was not statistically significant. On ROC curve analysis, nadir thrombocytopenia was found to be predictive of increased mortality (AUC-0.728) with a platelet count <77.35X109/L having a sensitivity of 67.7% and specificity of 74.3% in predicting higher mortality (Figure 3).
Variables
Thrombocytopenia At Admission(N=35)
95%Ci
No Thrombocytopenia At Admission(N=70)
95%Ci
P Value
Age in years
53.91 (49.1-58.6)
±4.78
57.64 (53.7-61.6)
±3.92
0.263
Male
21
49
Female
14
21
CRP in mg/L
126.08 (101.4-150.7)
±24.64
108.89 (88.8-128.9)
±20.05
0.185
SCVO2 in %
78.36 (73.3-83.3)
±4.97
76.01 (72.6-79.4)
±3.36
0.437
Lactates in mmol/L
2.94 (2.2-3.6)
±0.67
2.60 (2.07-3.1)
±0.53
0.111
Patients on MV
19
45
0.409
Patients on Ionotropes
18
35
0.778
28 d mortality
12
19
0.749
LOS in days
10.4 (8.2-12.6)
±2.24
10 (8.4-11.6)
±1.56
0.748
BSI
5
12
0.699
APACHE IV
70.55 (64.6-76.5)
±5.94
57.11 (53.2-61)
±3.89
0
Table 2: Comparison of clinical data in patients with and without thrombocytopenia (at admission).
Variables
Thrombocytopenia Nadir(N=63)
95%Ci
No Thrombocytopenia Nadir(N=42)
95%Ci
P Value
Age in years
55.65 (52.2-59.14)
±3.49
57.52 (53.6-61.4)
±3.92
0.560
CRP in mg/L
109.55 (90-129.1)
±19.58
122.24 (102.1-142.3)
±20.05
0.567
SCVO2 in %
76.96 (73.2-80.7)
±3.74
76.53 (73.1-79.9)
±3.36
0.883
Lactates in mmol/L
2.97 (2.5-3.5)
±0.52
2.34 (1.81-2.87)
±0.53
0.009
Patients on MV
41
23
0.242
Patients on Ionotropes
35
18
0.174
28 day mortality
25
6
0.016
LOS in days
11.16 (9.4-12.9)
±1.72
8.6 (7.0-10.1)
±1.56
0.039
BSI
18
11
0.805
APACHE IV
66.30 (61.7-70.9)
±4.59
54.33 (50.44-58.2)
±3.89
0.001
Table 3: Comparison of clinical data in patients with and without nadir thrombocytopenia.
Risk Factor Variables
Likelihood Ratio
P Value
BSI
0.181
0.430
MV
0.678
0.269
Ionotropes
0.079
0.471
Heparin use
1.481
0.176
Furosemide
0.964
0.618
Beta lactam
0.273
0.405
NSAIDS
0.00
0.589
antiplatelets
1.161
0.560
COMORBIDITIES
DM
0.00
0.608
COPD
0.00
0.684
CKD
0.525
0.345
Table 4: Risk Factors Associated with Thrombocytopenia in Sepsis.
Variables
Survivors (Mortality)(N=74)
95%Ci
Non Survivors(N=31)
95%Ci
P Value
SCVO2 (FX)
75.66 (72.4-78.92)
±3.26
79.4
±5.27
0.222
Lactates (EU)
2.473 (1.9-2.9)
±0.52
3.29
±0.74
0.007
APACHE IV (ID)
59.88 (56-63.7)
±3.86
65.34
±7.19
0.169
Thrombocytopenia admission (HB)
234.64(201.2-268.0)
±33.38
210.64
±49.59
0.440
Thrombocytopenia nadir (IV)
163.40 (136.7-190)
±26.66
84.64
±27.03
0.000
Table 5: Comparison of various prognostic markers with 28 day mortality.
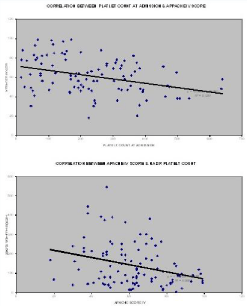
Figure 2: Correlation of admission thrombocytopenia and nadir thrombocytopenia with APACHE IV score.
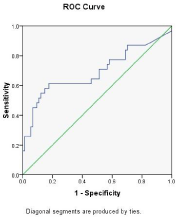
Figure 3: ROC curve analysis showing nadir thrombocytopenia as predictor
of mortality. AUC=0.728.
It was also seen that a platelet count drop by >33.62% had a sensitivity of 62.1% and specificity of 57.9% in predicting higher mortality on ROC curve (Figure 4). On comparing with APACHE IV as a marker for predicting mortality it was seen that drop in platelet count (AUC-0.692) had a slightly higher discriminative value for prediction of mortality than APACHE IV (AUC-0.586) on ROC curve (Figure 4).
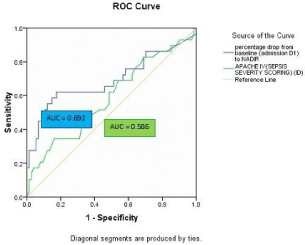
Figure 4: ROC curve analysis showing drop in platelet count and its
comparison with APACHE IV scores. Nadir thrombocytopenia has higher
discriminative value(AUC=0.692) than apache IV (AUC=0.586) in predicting
mortality.
After categorizing the decline in platelet count in four categories (<10%, 10%-30%, 30%-60%, >60%), kaplan-meier analysis was done which showed that greater the percentage decline in platelet count, lesser is the median survival time of the dying patients and hence association with higher mortality (Figure 5).
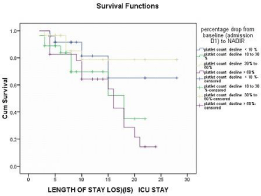
Figure 5: Kaplan-Meier estimates of hospital survival with the magnitude of
percentage decline in platelet count.
The LOS did not influence the prognostic impact of a decline in platelet count >33%.
Discussion
Thrombocytopenia is frequent in ICU [16]. At least one episode of thrombocytopenia was seen in 63 patients (60%) during their stay in ICU. A previous study by Schueren et al., showedthe prevalence of thrombocytopenia to be about 41% in a mixed ICU. Strauss et al. showed in his study that 44% developed ICU acquired thrombocytopenia during their stay in medical ICU. Perhaps, the reason for this difference was the constitution of the study group (surgical, medical, mixed, paediatric), their inclusion criteria (septic, trauma, <48hrs of ICU stay, shock) and the different criteria for thrombocytopenia (<200, <150, <100) taken in different studies [17]. In a similar Indian study, Sharma et al. found a comparable incidence of thrombocytopenia (55%) [20].
We analysed the association of admission thrombocytopenia, nadir thrombocytopenia and other admission prognostic markers like APACHE IV, CRP levels, ScvO2 and lactate levels with 28-day mortality (primary end point) and length of stay (secondary end point). Contrary to previous studies, [18] we found that the admission thrombocytopenia was not associated with higher 28-day mortality and the longer length of stay. However, nadir thrombocytopenia, high admission APACHE IV scores and high lactate levels were associated with higher mortality and longer length of stay. This supports the hypothesis that development of thrombocytopenia during stay but not the admission platelet count was a powerful predictor of mortality [18, 19].
As previously mentioned, thrombocytopenia in critically ill patients is often multifactorial, sepsis being the major contributing factor [7-10]. Other factors are disease severity as judged by higher SOFA score, low PaO2/FiO2, higher vasopressor dose, BSI, episode of CPR done and presence of Disseminated Intravascular Coagulation (DIC), use of medications like NSAIDS or heparin [19-24]. In our study, we found that none of the above mentioned factors have significantly contributed to the development of thrombocytopenia. Hence, low platelet count should be considered as an early indication of new onset sepsis rather than heparin or medication induced. It was also observed that lower the nadir platelet count achieved, higher is the mortality which has already been observed in previous study [19].
In our study, the cut-off value of thrombocytopenia on ROC curve which was associated with significant higher 28 day mortality was found out to be below 77.35x109/L.B. Sharma et al., in his study has found this figure to be 95x109/L [20]. Recently, the focus has been shifted from the absolute value of platelet count to the dynamic nature of thrombocytopenia, i.e.; the change of platelet count with time during the ICU stay. Many studies have shown that it is the percentage decline in platelet count or the blunted rise in platelet count after reaching its nadir that predicts the outcome better than just the absolute value [21].
In our study, decline in admission platelet count by 33.62% or more predicts higher mortality but not longer length of stay. Vanderschueren et al. [19] have shown that a fall by 50% in platelet count was strongly associated with mortality instead of absolute platelet count. In a large study conducted by Delphine et al. [22], they found that platelet count drop by 30% by day 4 is associated with a higher mortality and increased length of stay. Strauss also showed higher mortality with similar fall in platelet count by 30 % [23]. An Indian PICU study also confirmed that a fall by more than 27% had higher mortality as well as increased length of stay [24]. This difference from our study is seen mainly because of the inclusion criteria used where all the patients with admission thrombocytopenia were excluded.
This consolidates the previous theory that an early decline in platelet count needs active search and correction of the causative factors which most often is a new onset sepsis. Our study has many limitations which include a small sample size.The presence of multiple confounding factors at different times were difficult to control. All patients with stay of more than 48 hours were included in the study. This caused inclusion of a few patients with less than 4 days of stay which probably is not a sufficient time interval to see the platelet count trend. The total number of bleeding episodes and the number and type of blood and blood product transfusions given, which may have affected the platelet count were not recorded.
Conclusion
Thrombocytopenia < 150×109/cm3 in this subgroup of critically ill septic patients is common. No risk factors were found for its development in this study and hence, thrombocytopenia in ICU should be considered to be an independent marker of sepsis. The single absolute value, especially on admission carries little significance in predicting mortality. Nadir thrombocytopenia can be used as an independent predictor of mortality and also as a complementary marker to established prognostic markers like APACHE IV and lactate levels in septic patients. Percentage decline in platelet count by 33.62% predicts higher mortality. A daily estimation of platelet count and early recognition of a falling trend in it may be a cost effective and easily available modality to recognize the onset of sepsis and help in initiating of early and effective goal directed management of sepsis. However, whether this intervention based on platelet count improves mortality rate needs to be studied in larger prospective studies.
References
- Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest 1999; 115: 1363-1370.
- Crowther MA, Cook DJ, Meade MO, Griffith LE, Guyatt GH, Arnold DM, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005; 20: 348-353.
- Nguyen HB, Rivers EP, Abrahamian FM, Moran GJ, Abraham E, Trzeciak S, et al. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med 2006; 48: 28-54.
- Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001; 29: 99-106.
- Rivers EP, Mcintyre L, Morro DC, Rivers KK. Early and innovative interventions for severe sepsis and septic shock: taking advantage of a window of opportunity. 2005; 173: 1054-1065.
- Stéphan F, Thiolière B, Verdy E, Tulliez M. Role of hemophagocytic histiocytosis in the etiology of thrombocytopenia in patients with sepsis syndrome or septic shock. Clin Infect Dis. 1997; 25: 1159-1164.
- Corrigan JJ Jr, Ray WL, May N. Changes in the blood coagulation system associated with septicemia. N Engl J Med. 1968; 279: 851-856.
- Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993; 104: 1243-1247.
- Bogdonoff DL, Williams ME, Stone DJ. Thrombocytopenia in the critically ill patient. J Crit Care 1990; 5: 186-205.
- Vandijck DM, Blot SI, De Waele JJ, Hoste EA, Vandewoude KH, Decruyenaere JM. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung. 2010; 39: 21-26.
- Nijsten MW, TenDuis HJ, Zijlstra JG, Porte RJ, Zwaveling JH, Paling JC, et al. Blunted rise in platelet count in critically ill patients is associated with worse outcome. CritCare Med. 2000; 28: 3843-3846.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/ SIS International Sepsis Definitions Conference. Crit Care Med. 2003; 31:1250-1256.
- Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care. 2008; 12: 161.
- Unertl K, Kottler BM. [Prognostic scores in intensive care]. Anaesthesist. 1997; 46: 471-480.
- Jack E Zimmerman, Andrew A. Kramer et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Critical Care Med. 2006; 34: 1297-1305.
- Hanes SD, Quarles DA, Boucher BA . Incidence and risk factors of thrombocytopenia in critically ill trauma patients. Ann Pharmacother. 1997; 31: 285-289.
- Cawley MJ, Wittbrodt ET, Boyce EG, Skaar DJ. Potential risk factors associated with thrombocytopenia in a surgical intensive care unit. Pharmacotherapy. 1999; 19: 108-113.
- Bonfiglio MF, Traeger SM, Kier KL, Martin BR, Hulisz DT, Verbeck SR. Thrombocytopenia in intensive care patients: a comprehensive analysis of risk factors in 314 patients. Ann Pharmacother. 1995; 29: 835-842.
- Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000; 28: 1871-1876.
- Sharma B, Sharma M, Majumder M, Steier W, Sangal A, Kalawar M. Thrombocytopenia in septic shock patients-a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care 2007; 35: 874-880.
- Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002; 30: 753-756.
- Moreau D, Timsit JF, Vesin A, Garrouste-Orgeas M, de Lassence A, Zahar JR, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007; 131: 1735-1741.
- Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002; 30: 1765-1771.
- Agrawal S, Sachdev A, Gupta D, Chugh K. Platelet counts and outcome in the pediatric intensive care unit. Indian J Crit Care Med. 2008; 12: 102-108.