
Case Report
Austin Crit Care J. 2016; 3(2): 1018.
Extracorporeal Membrane Oxygenation for Disseminated Herpes Simplex Virus Infection with Multiorgan Failure
Triplet RDO¹*, Al-Ibrahim OMD² and Kramer BDO²
¹Department of Pediatrics, Statue University of New York at Buffalo School of Medicine and Biomedical Sciences, Women and Children’s Hospital of Buffalo, USA
²Department of Pediatrics, Statue University of New York at Buffalo School of Medicine and Biomedical Sciences, USA
*Corresponding author: Triplet R, Department of Pediatrics, Statue University of New York at Buffalo School of Medicine and Biomedical Sciences, Women and Children’s Hospital of Buffalo, 219 Bryant St., Buffalo, NY 14222, USA
Received: June 08, 2016; Accepted: July 26, 2016; Published: July 27, 2016
Abstract
Neonatal disseminated Herpes Simplex Virus (HSV) infection is a devastating disease causing rapidly progressive multiple organ failure and death, often despite aggressive antiviral treatment and intensive care support. Respiratory and hemodynamic instability requires intensive care, with a smaller sub-set of patients needing to be supported by extracorporeal techniques. Previously, neonates with HSV infection requiring Extracorporeal Membrane Oxygenation (ECMO) had a 75% mortality rate. Due to the high mortality rate of neonates with disseminated HSV, current recommendations are to not place patient’s with HSV onto ECMO.
MS is a 7 day old male who presented hypothermia and hypoglycemia. He was determined to be in shock and was admitted to the Pediatric Intensive Care Unit. Within the first 12 hours of presentation, the patient continued to decompensate with worsening hypoxia despite escalation of ventilator support. Patient was cannulated for VA ECMO which resulted in improved oxygenation. He was started on prophylactic antibiotics of vancomycin, cefotaxime, and acyclovir pending culture results. On day 2 of admission, the nasopharyngeal swab for HSV came back positive for HSV type 1 and he was diagnosed with disseminated HSV. The patient remained on ECMO for a total of 7 days and was successfully decannulated. The patient was discharged home after a one month hospitalization with a normal exam at the time of discharge.
This case challenges the idea of HSV as a contraindication to ECMO. Had the diagnosis been known at the time of cannulation, based on current recommendations this patient would not have been placed on ECMO. Placing the patient on ECMO gave the team more time to treat the underlying cause of his septic shock and subsequent arrest. Timely placement of these patients onto ECMO with appropriate treatment of the underlying issue could lead to improved survival of neonates with disseminated HSV.
Keywords: Herpes simplex virus; Extracorporeal membrane oxygenation
Abbreviations
HSV: Herpes Simplex Virus; ECMO: Extracorporeal Membrane Oxygenation; VA: Venoarterial; ELSO: Extracorporeal Life Support Organization; PICU: Pediatric Intensive Care Unit; iNO: inhaled Nitric Oxide; FFP: Fresh Frozen Plasma
Case Report
Herpes Simplex Virus (HSV) is an enveloped, double stranded, DNA virus which belongs to the herpes virus family and causes significant morbidity and mortality in the neonatal period [1-3]. The majority of neonatal HSV infections is acquired during delivery, although in-utero and postnatal infections do occur. Disseminated neonatal HSV infection is a devastating disease causing rapidly progressive multiple organ failure and death. This progression of organ failure and death often occurs despite aggressive antiviral treatment and intensive care support [1].
Respiratory and hemodynamic instability associated with disseminated HSV infections frequently requires the neonate to be cared for in the intensive care unit. There is a smaller sub-set of these patients who develop worsening cardio-respiratory compromise and may need to be supported by extracorporeal techniques. In a review by Meyer of the Extracorporeal Life Support Organization (ELSO) registry from 1988-1994, 13 patients with disseminated HSV were placed on extracorporeal membrane oxygenation (ECMO) with a 31% survival rate, however the impact of variables such as patient comorbidities were not systemically assessed [4].
Prodhanalso reviewed the ELSO registry from 1985-2005and looked at neonates with HSV infection requiring ECMO. This study looked at death as a primary outcome. The review found a 100% mortality rate of neonates with disseminated HSV on ECMO at his institution and an overall mortality rate of 75% [3]. Due to the high mortality rate of neonates with disseminated HSV, current recommendations are to not place patient’s with HSV onto ECMO. We report a case of a 7 day old male who presented to the Emergency Room with hypothermia. The parents reported that the patient had a history shallow breathing two days prior to arrival that self-resolved. Later that same night, after the breathing concerns had resolved, the patient was noted to have decreased oral intake, increased fussiness, and return of the shallow breathing. The next morning the patient was seen by a pediatrician but had a normal examination and no intervention was warranted. The following evening a rectal temperature was obtained and the baby was found to be hypothermic at 96.5oF. The patient’s fingernails and lips were then noted to turn blue and he seemed less responsive. At this point, he was brought to the emergency room where he was noted to be in shock. His initial vital signs were a temperature of 29oC, a heart rate of 70 with pulse oximeter of 60%. The initial laboratory values revealed a pH of less than 6.8, a PCO2 of 110 mmHg, PvO2 of 66 mmHg, bicarbonate of 15mmol/L, a base excess of -20.4and a glucose of 13 mg/dL. He was noted to have a significantly elevated liver enzymes and prolonged coagulation profile. Upon presentation, he had an AST of 1345 units/L, ALT of 192 units/L, Bilirubin of 8.3 mg/dL, PT of 33.7 seconds, PTT of 83.7 seconds, and an INR of 3.45. His lactate was elevated at 5.9 mmol/L. After the patient was emergently intubated, adequately fluid resuscitated, and received medications to correct his underlying acidosis and electrolyte abnormalities; he was transferred to the Pediatric Intensive Care Unit (PICU).
While in the PICU, the patient required vasopressor support with both dopamine and epinephrine for severe hypotension from septic shock. He continued to have worsening hypoxia and hypercarbia, with development of new onset pulmonary edema. sDespite aggressive therapy with high mean airway pressure and inhaled nitric oxide (iNO), the patient’s pulse oximetry readings remained less than 50%. His oxygen index continued to rise to a peak level of 155. Given the patient’s worsening hypoxia, acidosis, and potential for cardiac arrest, the decision was made to place the patient on ECMO. The patient was cannulated for VA ECMO andboth the PO2 and PCO2 significantly improved (Figures 1 and 2).
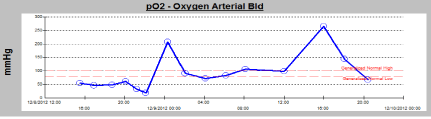
Figure 1: PaO2 levels in patient before and after initiation of ECMO.
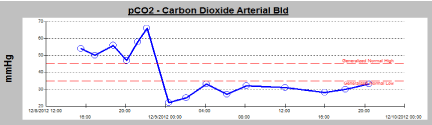
Figure 2: PaCO2 levels in patient before and after initiation of ECMO.
The patient was successfully cannulated with a 12 French venous cannula and an 8 French arterial cannula without complications. Post cannulation imaging showed the venous cannula to be in the right atrium and the aortic cannula to be in good position in the transverse aortic arch. After slowly increasing the flows on the ECMO circuit to full flows of 75 mL/kg/min, the saturations increased to 98%. Throughout his ECMO course, he had no difficulties maintaining a flow rate of 75 mL/kg/min. In addition, the patient’s hemodynamic status improved after initiation of ECMO and all vasopressor support was discontinued within one hour. His initial echocardiogram showed pulmonary hypertension that resolved by day 6 of ECMO.
A partial sepsis workup was performed at the time of admission that included a complete blood count and blood and urine cultures. CBC revealed a white cell count of 28.4 x109, Hgb of 13.9 g/dL, Hct of 42.1%, and a platelet count of 85 x109 with a bandemia of 38%. Antibiotic and antiviral medications were started upon admission in response to the CBC results. A lumbar puncture was not initially performed as the patient was not hemodynamically stable enough to undergo the procedure. Nasopharyngeal and rectal swabs were sent to test for herpes simplex virus. Throughout his hospital course, both blood and urine culture remained negative. The nasal swab from admission was positive for HSV type 1. A lumbar puncture was eventually performed on day nine of acyclovir therapy, once the patient was stable off ECMO, and the HSV PCR done at that time was positive for HSV. A repeat lumbar puncture was performed on day 20 of acyclovir therapy and the CSF HSV PCR was noted to be negative at that time. The patient completed a 21day course of acyclovir.While on ECMO, he had serial head ultrasounds that showed no intracranial hemorrhage. His daily neurologic examination was initially normal and continued to be reassuring without changes throughout the course of his ECMO therapy. Patient initially presented with oliguria and then anuria within 24 hours of cannulation. He required continuous renal replacement therapy while on the ECMO circuit for his acute kidney injury. Patient developed liver failure within 12 hours of presentation. After the initiation of antiviral therapy and subsequent ECMO cannulation, the liver enzymes slowly improved. By the end of therapy, his liver failure had resolved.His coagulopathy persisted on ECMO, likely as a result of his continuous anticoagulation. He required multiple transfusions of red blood cells and platelets each day. As a result of his ongoing DIC, he required a fresh frozen plasma infusion (FFP) for the first 5 days of ECMO. After his FFP infusion was discontinued, he continued to require intermittent transfusions with FFP until the day of decannulation.After discontinuation of ECMO, the patient’s coagulopathy resolved.
The patient remained on VA ECMO and did not develop any complications, clots in his ECMO circuit; intracranial bleeding, cannula sight bleeding; or intracranial ischemic insult, while on ECMO. He gradually improved with antiviral therapy and was successfully decannulated after 8 days. He continued to demonstrate a steady clinical improvement and was eventually discharged to home after being hospitalized for one month. At the time of discharge, he had a normal physical exam with no signs of neurologic deficits.
This case challenges the idea that HSV is a contraindication to ECMO. Had the diagnosis been known at the time of cannulation, based on current recommendations this patient would not have been placed on ECMO. This patient received appropriate treatment for his HSV while on ECMO and was successfully decannulated with no known sequela. Placing the patient on ECMO gave the team more time to treat the underlying cause of his septic shock and subsequent respiratory arrest. If this patient had not been placed on ECMO, it is suspected that he would have died from his overwhelming sepsis within the first twenty four hours of his admission. Timely placement of these patients onto ECMO with appropriate treatment of the underlying issue could lead to improved survival of neonates with disseminated HSV.
References
- Prodhan P, Wilkes R, Ross A, Xiomara Garcia, Adnan T Bhutta, Peter Rycus et al. Neonatal Herpes Virus infection and Extracoporeal Life Support. Pediatric Critical Care Medicine. 2010; 5: 599-602.
- Whitley R, Arvin A, Prober C, Corey L, Burchett S, Ploktin S, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. New England Journal of Medicine. 1991; 324: 450- 454.
- Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatric Infect Dis. 2005; 16: 271-281.
- Meyer TA, Warner BW. Extracoporeal life support for the treatment of viral pneumonia: collective experience from the ELSO registry. Journal of Pedatric Surgery. 1997; 32: 232-236.