
Research Article
Austin Crit Care J. 2019; 6(1): 1028.
Impact of a Nurse-Implemented Sedation and Analgesia Algorithm on Complications of Critical Illness in a Surgical Intensive Care Unit
Pottier V*, Bornemann Y, Fauroux J, Desmeulles I, Verrier P, Orabona M, Samba D, Viquesnel G, Ethuin F and Hanouz JL
Department of Anesthesia and Surgical Intensive Care, Caen University Hospital, France
*Corresponding author: Véronique Pottier, M.D.Département d’Anesthésie-Réanimation (niveau 6), CHU de Caen, avenue Côte de Nacre, 14033 Caen Cedex, France
Received: October 09, 2019; Accepted: November 11, 2019; Published: November 18, 2019
Abstract
Objective: To examine the effects of a nurse-applied sedation and analgesia algorithm on sedative doses, duration of mechanical ventilation (MV), patient comfort, morbidity and mortality.
Design: Before-and-after prospective, observational study.
Setting: 26-bed Surgical ICU in Caen University Hospital.
Patients: Mechanically ventilated patients with sedation predicted to last 48 hours and without brain injury, between November 2014 and April 2017.
Intervention: Setting up an algorithm considered as recommended common practice.
Measurements and Main Results: A total of 1156 mechanically ventilated patients were admitted during the study period. Among the 145 eligible patients, 100 were included during a « Before » period and 45 during an « After » period. The duration of MV after inclusion was significantly shorter in the « After » period (11 vs 8 days, p = 0.042), as the duration of target RASS (-2 to 0) was significantly longer (0 vs 1 day, p = 0.038), the duration of RASS › - 2 was significantly shorter (7 vs 3 days, p ‹ 0.001), and the dose of sedatives was significantly decreased (1330 vs 315 mg, p ‹ 0.001 for hypnotics and 1803 vs 900 μg, p ‹ 0.001 for opioids, respectively) along with the sedation cost (25 vs 12 euros, p = 0.004). The patients experienced less ventilator-acquired pneumonia (VAP) and delirium during the « After » period (55% vs 24%, p = 0.004, and 41% vs 27%, p = 0.015, respectively).
Conclusions: The nurses’ implementation of a sedation-analgesia algorithm was associated with a trend towards a reduction in duration of MV and ICU length of stay. Moreover, the prevalence of VAP and delirium was reduced. This type of algorithm is necessary to reduce the morbidity and mortality associated with MV.
Keywords: Sedation; Analgesia; Algorithm; Intensive care unit; Ventilatoracquired pneumonia; Delirium
Introduction
In intensive care units (ICU), the main objectives of sedation and analgesia are to ensure the safety of critically ill patients during therapeutic and diagnostic procedures, as well as their physical and psychological comfort. Furthermore, sedation and analgesia are an important part of treatment during the acute phase of life-threatening illness, such as acute respiratory distress syndrome, severe neurologic aggression, and shock [1].
To limit the risks associated with sedation and analgesia, [2,3]. Standardized and repeated evaluation of efficacy and adequacy is mandatory at every stage of the patient’s clinical evolution [4]. This recommendation is widely accepted by physicians and based on validated clinical scales such as the Richmond Agitation-Sedation Scale (RASS) [5,6] scale and the Behavior Pain Scale (BPS) [7] quantifying consciousness, pain, and comfort in patients with mechanical ventilation (MV).
Thus, implementation of a dynamic sedation and analgesia algorithm has been shown to improve drug administration, patient comfort, awakening and cooperation. Because of their permanent presence next to patients, the transfer of the management of sedationanalgesia to nurses should result in a fine adaptation of the dosages without significant mismatch or disruption. This practice was initiated by Kollef et al in 1998, [8] followed by Kress et al in 2000 [9]. and adopted in US4 and French1 guidelines. Indeed, these studies showed a decreased duration of MV and length of stay in the ICU. However, the studies were carried out in medical or polyvalent ICUs [10,11]. Which are not representative of a surgical ICU. This is of importance, since it has been shown that patients may differ substantially between medical and surgical ICUs [12].
Consequently, the aim of the present study was to examine effects of the implementation of a nurse-controlled sedation and analgesia algorithm on sedative drug consumption reduction, major clinical outcomes, and patient comfort in a surgical ICU.
Materials and Methods
We conducted a prospective, « Before-After » interventional study in the 26-bed surgical ICU of Caen University Hospital between November 2014 and April 2017. The study was approved by the local Ethical Committee (CPP Nord Ouest III, CHU de Caen, Caen, France) under the number A14-D65-VOL.23, on December 06, 2014. The committee considered it as part of routine practice, and patient approval was not required. However, written information was systematically given to each included patient or to their next of kin. The study is recorded in ClinicalTrials with the number NCT03186521.
Inclusion criteria`
All patients aged 18 years or older, admitted to the ICU, and anticipated to require more than 48 hours of sedation and analgesia were eligible and assessed for enrollment.
Non-inclusion criteria
Patients were not eligible if they were under guardianship or ‹ 18 years; pregnant; under palliative care; experiencing brain injury; presenting with an initial Glasgow Coma score ‹ 14; receiving neuromuscular blocking agents at the time of enrollment; under therapeutic sedation (Acute Respiratory Distress Syndrome, acute severe asthma, intracranial hypertension, etc.); or admitted following resuscitated cardiac arrest.
Exclusion criteria
Patients were excluded from the study if they were extubated or dead within 48 hours after inclusion.
Study protocol
Patients admitted to the surgical intensive care unit with orotracheal intubation and mechanical ventilation were recorded by one of the two principal investigators (VP and YB).
« Before » Period: Sedation and analgesia was exclusively managed by the attending physician, guided by the Richmond Agitation-Sedation Scale (RASS) [5,6] and Behavior Pain Scale (BPS) [7] and recorded every 4 hours by nurses. The dosage and choice of the hypnotic drug (Propofol® or Midazolam®) was at the discretion of the physician, and the only opioid used was Sufentanil®.
Between the two periods, principal investigators conducted several informational meetings with the paramedical teams and the medical staff.
« After » Period: Following medical prescription, the patient’s sedation and analgesia was managed by the ICU nurses according to the protocol displayed in each bedroom (see Appendix 1).
A flow chart of the study design is depicted in Figure 1. During the study period, 1156 intubated and mechanically ventilated patients were admitted to the surgical ICU of Caen University Hospital. Among the 1014 non-eligible patients 507 (50%) were sedated less than 48 hours and 304 (30%) had an initial Glasgow coma score ‹ 14.
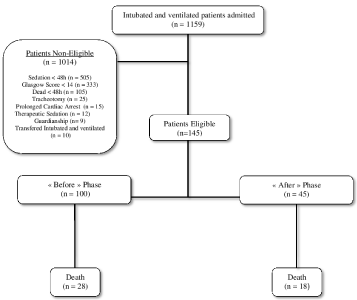
Figure 1: Flow chart of the study.
Among the 145 eligible patients (12.5%), 100 were included during the « Before » period and 45 during the « After » period.
Data collection
The anonymized data were recorded on an eCRF platform based on OpenClinica® (OpenClinica, LLC, Waltham, Massachusetts, USA).
Data recorded were as follows:
Patient demographic characteristics: Age, sex, body mass index, medical and surgical history, tobacco consumption, reason for ICU admission and simplified acute physiology score (SAPS II) [13].
pData related to mechanical ventilation: ICU and hospital length of stay, total duration of MV (primary end-point), duration of MV after inclusion, duration of sedation, duration of « comatose » (defined as the time between cessation of sedative drugs and response to simple orders), duration of weaning from mechanical ventilation (defined as the time between the first spontaneous breathing challenge and extubation) (see Appendix 2).Data related to sedation: Sedation was evaluated by the RASS recorded at inclusion, the number of days with an RASS between -2 and 0, the number of days with an RASS › -2; analgesia was evaluated by the BPS at inclusion, the number of days with a BPS ‹ 5 and with a BPS › 5, cumulative doses of sedative drugs (hypnotics, opioids and neuromuscular blocking drugs), and the cost of sedation according to the total doses in euros (€).
Clinical outcomes: Occurrence of ventilator-acquired pneumonia (VAP), [14] re-intubation, use of non-invasive ventilation or tracheotomy, gastrointestinal injury defined as the occurrence of digestive hemorrhage or peritonitis, occurrence of delirium according to the Confusion Assessment Method for Intensive Care Unit (CAMICU) [15]. duration of delirium, need for neurologic explorations for delayed awakening (excessive duration of comatose without hypnotics), self-extubation; occurrence of cardiac and hemodynamic failure, duration of vasopressors and/or positive inotropic drugs, need for renal replacement therapy, Sequential Organ Failure Assessment (SOFA) [16] score at D0, D3, D7, D14, D28, and ICU discharge, and death in ICU.
Statistical analysis
The number of patients required was calculated based on a 50% decrease in the duration of mechanical ventilation (primary endpoint of the study) during the « After » period. In our surgical ICU, the mean duration of mechanical ventilation was 10 ± 1 days. Setting the alpha risk at 5% and the beta risk at 90%, the number of patients required was 100 in each period.
Quantitative data are expressed as the mean ± SD, or median with confidence intervals at 95% [CI 95%], according to the normality of their distribution, and were compared using the Mann–Whitney non-parametric U test when data were not normally distributed, or Student’s t-test for normally distributed data. Categorical data are expressed as percentages and were compared using the Fisher’s exact test when data were not normally distributed, or the Chi-square test for normally distributed data.
The normality of the distribution was determined by the Agostino Pearson test. The level of significance was set at p ‹ 0.05 between the two periods tested (Before/After).
All statistical analyses were performed with software R 3.4.0: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing©, Vienne, Austria).
Results
Patients characteristics
pDemographic and main characteristics of the patients are reported in Table 1. There were no significant differences between the two periods, except for tobacco consumption, which was lower in the « After » period (39 ± 24 vs 25 ± 14, p = 0.033).
Baseline Characteristics
BEFORE Period
(n = 100)a
AFTER Period
(n = 45)b
p Value
Age (years)
61 ± 20
65 ± 14
0.177
Male gender
68 (68%)
38 (84%)
0.062
BMI
29 ± 8
28 ± 6
0.328
Underlying diseases
- Psychiatric illness
16 (16%)
8 (18%)
0.711
Psychotropic treatment
14 (14%)
7 (16%)
1.000
- Chronic alcoholism
25 (25%)
14 (31%)
0.259
Number of drinks per day
3.6 ± 1.8
5.7 ± 3.8
0.447
- Chronic smoking
60 (60%)
21 (47%)
0.415
Number of packs-years
39 ± 24
25 ± 14
0.033
Reason for ICU admission
Respiratory distress
26 (26%)
7 (16%)
0.202
Shock
46 (46%)
25 (56%)
0.369
Trauma
7 (7%)
4 (9%)
0.738
Scheduled surgery
7 (7%)
3 (7%)
1.000
Other
14 (14%)
6 (13%)
1.000
SAPS II
49 [38; 61]
53 [38; 64]
0.622
Table 1: Baseline characteristics according to before and after Periods.
Data related to mechanical ventilation and sedation
Table 2 reports data on MV and sedation during the ICU stay. The ICU length of stay was shorter in the « After » period (26 [16;36] vs 16 [12;30], p = 0.059).
Clinical Characteristics
BEFORE Period
(n = 100)a
AFTER Period
(n = 45)b
p Value
ICU length of stay (days)
26 [16; 36]
16 [12; 30]
0.059
Hospital length of stay (days)
47.5 [30; 62]
39 [25; 57]
0.251
Related to Mechanical Ventilation (MV)
Duration of total MV (days)
15 [9; 22]
11 [8; 17]
0.140
Duration of MV before inclusion (days)
1 [1; 2]
1 [0; 3]
0.286
Duration of MV after inclusion (days)
11 [7; 18]
8 [5; 11.5]
0.042
Duration of sedation (days)
7 [5; 14]
7 [3; 9]
0.042
Duration of Comatose (days)
1 [0; 3]
0 [0; 2]
0.102
Duration of weaning from MV (days)
3 [1; 7]
1.5 [1; 5]
0.063
Related to Sedation
RASS at inclusion
-5 [-5; -4]
-5 [-5; -4]
0.311
Target RASS (-2 to 0) (days)
0 [0; 2]
1 [0; 3]
0.038
RASS > -2 (days)
7 [4; 12]
3 [2; 5]
< 0.001
BPS at inclusion
3 [3; 3]
3 [3; 3]
0.430
Target BPS (< 5) (days)
7 [4; 14]
5 [3; 7]
0.002
BPS > 5 (days)
0 [0; 0]
0 [0; 0]
0.902
Total cumulative doses
Midazolam (mg) Propofol (mg)
Opioids (μg) Neuromuscular blocking agents (mg)
1330 [518; 2544]
0 [0; 4976]
1803 [1098; 4290]
0 [0; 50]
317 [15; 720]
0 [0; 10360]
900 [450; 1680]
0 [0; 125]
< 0.001
0.514
<0.001
0.309
Cost of sedation (€)
25 [13; 67]
12 [7; 34]
0.004
Table 2: Clinical characteristics related to mechanical ventilation and sedation.
The total duration of MV was not significantly different between the « Before » and « After » periods (15 [9;22] vs 11 [8;17], p = 0.140).
The durations of MV after inclusion and sedation were significantly shorter during the « After » period (11 [7;18] vs 8 [5;11.5], p = 0.042, and 7 [5;14] vs 7 [3;9], p = 0.042, respectively). Consumption and cost related to sedation and analgesia were decreased during the « After » period (1330 vs 315 mg, p ‹ 0.001 for hypnotics and 1803 vs 900 μg, p ‹ 0.001 for opioids, respectively; 25 vs 12 euros, p = 0.004 for the cost).
Data related to complications of critical illness
Table 3 shows clinical outcomes recorded during the ICU stay.
Complications of Critical Illness
BEFORE Period
(n = 100)a
AFTER Period
(n = 45)b
p Value
Respiratory Failure
67 (67%)
22 (54%)
0.093
VAP
55 (55%)
11 (24%)
0.004
Replacement of Endotracheal tube
23 (23%)
17 (38%)
0.654
Time limit of reintubation (days)
3 [0.5; 7.5]
4 [1.5; 10]
0.476
NIV
37 (37%)
18 (40%)
0.751
Tracheotomy
4 (4 %)
2 (4 %)
1.000
Gastro-intestinal Injury
39 (39%)
17 (38%)
1.000
Neurological Injury
51 (51%)
24 (53%)
0.093
Delirium (CAM-ICU +)
41 (41%)
12 (27%)
0.015
Duration of delirium (days)
5 ± 4
7 ± 8
0.548
Self-extubations, tear off devices, etc.
9 (9%)
6 (13%)
0.558
Resort to cerebral imaging for wake-up delay
4 (4%)
2 (4%)
1.000
Cerebral Scan
0
2 (4%)
0.067
MRI
1 (1%)
1 (2%)
1.000
EEG
4 (4%)
1 (2%)
0.333
Cardiac and/ or Hemodynamic
88 (88%)
38 (84%)
1.000
Duration of catecholamines (days)
7 [4; 11.5]
5 [3; 10]
0.416
Renal Injury
66 (66%)
35 (78%)
0.098
Resort to RRT
50 (50%)
25 (56%)
0.66
SOFA Score
D0
7 [5; 10]
9 [6; 11]
0.088
D3
6 [3; 9]
6 [4; 10]
0.474
D7
4 [2; 7]
4 [2; 8]
0.579
D14
4 [1; 7]
3.5 [1; 7]
0.982
D28
3 [1; 7]
5 [3; 7.5]
0.114
ICU discharge
0 [0; 1]
1 [0; 2]
0.071
Death
28 (28 %)
18 (40 %)
0.228
Table 3: Patient characteristics related to complications of critical illness.
Adverse events related to an inappropriate depth of sedation were not different between the two periods.
The occurrence of VAP and delirium were significantly decreased during the « After » period (55% vs 24%, p = 0.004, and 41% vs 27%, p = 0.015, respectively). There was no significant difference in mortality between the two periods.
Discussion
The present study showed that implementation of a sedation analgesia protocol in a surgical ICU improved the management of sedation and decreased the duration of MV after inclusion, the incidence of VAP and delirium. Furthermore, the decrease in hypnotic drugs and opioid consumption resulted in a 50% decrease in cost of sedation analgesia.
Complications associated with excessive sedation negatively impact the morbidity and mortality of patients. [2,3,8] Kollef et al, were the first to showed in 1998 that patients receiving intravenous continuous sedation had durations of MV, ICU and hospital lengths of stay longer than those who did not receive sedation or intermittent sedation.8 Subsequently, several studies have shown that sedation protocols managed by the nursing team reduced the duration of MV [17-19].
Our study is far from being the first to be reported, but few studies have been carried out in an exclusively surgical ICU, and few have reported on the serious adverse effects that excessive sedation can induce, such as the occurrence of VAP [19,20].
In contrast to the previously mentioned precursors, but similar to some others, [21,22] we were unable to demonstrate a significant difference regarding duration of MV in patients with sedationanalgesia protocol managed by the nursing team, according to objectives of RASS and BPS, compared to those whose sedation was managed by the medical team outside of any protocol. Frawley et al. [23] showed a decreased mean duration of MV in a retrospective study from before to after the introduction of a new sedation protocol. They changed the first-line analgesic/sedative agents from pharmacokinetic long-acting agents to pharmacokinetic brief-acting agents. Perhaps we should not have left the choice of the hypnotic (Midazolam® or Propofol®) to the discretion of the physician because Midazolam® was more widely chosen, although there was a significant difference in its use between the « Before » and « After » periods. Another mistake might have been not to change the opioid between the two periods.
However, the duration of MV after inclusion was significantly shorter in the « After » group (11 [7, 18] vs 8 [5, 11.5], p = 0.042). Moreover, there is a trend toward a reduced ICU length of stay (10 days) in patients of equivalent severity (see SAPS II). Similarly, the duration of sedation was significantly shorter in the « After » group and correlated with doses of sedatives as well as cost. The target RASS (-2 to 0) was significantly longer, and the RASS « exceeded » (› - 2) shorter in the « After » group. The duration of weaning from MV also showed a downward trend in the « After » group. All of this contributes to suggesting a reduction in the morbidity and mortality of patients and a considerable benefit in terms of hospital economics.
In fact, the introduction of and adherence to the A (Assess, prevent and manage pain) B (Both awakening and spontaneous breathing trial coordination) C (Choice of analgesic and sedation) D (Delirium: assess, prevent and manage) E (Early mobility and exercise) F (Family engagement and empowerment) bundle [24] is associated with improved patient-centred outcomes such decreased MV [25,26], hospital length of stay and so hospital cost [25], as well as improved ICU readmission rates and discharge disposition of ICU survivors [26].
Nevertheless, the A was not easy to achieve in our study because if the implementation of the protocol was beneficial regarding some parameters without major adverse events, there was a parameter that was worse in the 2nd period: BPS. There is a balance to obtain, which is not easy.
The objective of implementing a sedation protocol is to decrease the duration of MV and, consequently, the risk of VAP associated with it [27]. This disease, with its significant mortality and considerable morbidity, particularly in terms of prolonging ICU and hospital length of stay, is relatively frequent [28,29]. Thus, in our study, 66 patients (45.5%) experienced VAP, which is in line with the literature. However, in the « After » group, patients whose total duration of MV was shorter by 3.5 days, only 11 (24.4%) patients experienced VAP compared to 55 (55%) in the « Before » group (p = 0.004). This result is consistent with the results of Quenot et al., who showed a decrease in the VAP rate from 15 to 6% (p = 0.005), while the duration of MV decreased from 8 [2.2-22] to 4.2 [2.1-9.5] days (p = 0.001) following the implementation of a sedation protocol managed by nurses [30].
Therefore, the reduction of doses and duration of sedation and MV should be a constant objective, although the current recommendations include avoiding sudden withdrawal of drugs in order to limit the risk of withdrawal syndrome [1,31]. However, its incidence remains high, varying from 11 to 80% according to other studies. Ouimet et al., in a prospective observational study dating from 2007, estimated withdrawal at 31.8%. The use of sedationanalgesia multiplied the risk of experiencing delirium by 3.2 [1.5- 6.8] [32]. In our study, the incidence of delirium was 36.5%, but the implementation of the protocol allowed a significant reduction of its incidence, as well as the recent studies on the ABCDEF bundle [26,33].
Similar to the studies cited above, it is important to note that in our study, there was no significant difference between the two groups regarding the occurrence of adverse events due to an inappropriate depth of sedation, or even a tendency to decrease them. Therefore, it is safe to entrust the management of sedation to the paramedical team according to a previously established protocol.
Finally, the studies all raise the importance of a regular monitoring of the state of vigilance, pain and confusion of critically ill patients.
Our study has several limitations, which must require prudent conclusions and interpretations to be made. First, it was a monocentric study.
Second is the lack of power; the study was interrupted for logistical reasons before reaching the number of subjects needed to demonstrate a significant result. Indeed, there is only a tendency to decrease the duration of MV by 3.5 days.
Third, the sedation protocol may not be optimal, for example, by a greater reduction in sedative drugs at each assessment in order to achieve the RASS objectives more quickly. The principal investigators were able to note that a low RASS score did not systematically result in a corresponding decrease in the administered doses. This weak adherence to the sedation protocol has already been noted, and other studies proposed suggesting the participation of a pharmacist [34] or focusing on initial team education [35,36]. Nevertheless, our teams had been using the RASS and BPS scores for more than 3 years at the start of the study, and ten meetings were held at the protocol implementation and before the « After » period. We can assume that the nurse/patient ratio was too low in our service and did not always allow dose changes when the RASS was excessive. Sometimes, a different nurse alert the nurse of the excessive RASS if the nurse in charge of the patient is busy, but without touching the syringes, just to move the protocol forward. We can also point out their timidity in regard to having more responsibilities: they are not all motivated in the same way. Nevertheless, the difference in RASS was significant even if the practices were imperfect and are perfectible.
The fourth limitation is the non-randomized nature of the study, which may limit its impact; the two groups did not show any significant difference on the criteria studied, except for the significantly lower consumption of tobacco in the « After » group, which may have a beneficial impact on the occurrence of delirium. Indeed, a history of active smoking is an independent factor of agitation in intubated patients, as seen in Lucidarme et al. in 2010 [37].
Conclusion
This study did not demonstrate a reduction in the duration of MV when the « comfort » sedation of ICU patients was managed by the nursing team using a protocol. However, ICU length of stay had a tendency to decrease, correlated with a significant decrease in sedative drugs and their cost and possibly associated with a significant reduction in incidence of VAP and delirium.
Moreover, the association of a daily interruption of sedation with the implementation of a protocol, 9, 11, [38] the decline of benzodiazepines in favor of Dexmedetomidine® [31,39,40], or respect for the sleep-wake cycle [41] or music therapy, are all research tracks that invite us to carry out complementary studies aimed at improving the prognosis and the experience of patients admitted to the ICU, which is a source of reduction in post-traumatic stress disorder [42] and in mortality [43].
Acknowledgment
In addition to the authors of this article, we thank all other physicians in the Surgical ICU for their participation in the data collection. We also thank the nursing staff, residents, and secretaries of the intensive care unit for their cooperation with the protocol.
A particularly great thanks to the Department of Clinical Research, Pr Jean-Jacques Parienti for the opening of the E-crf, Jean- Jacques Dutheil for the making of the booklets.
Conflict of Interest and Source of Funding
The authors have no conflicts of interest to declare.
References
- Sauder P, Andreoletti M, Cambonie G, Capellier G, Feissel M, Gall O, et al. Sedation and analgesia in intensive care (with the exception of new-born babies). French Society of Anesthesia and Resuscitation. French-speaking Resuscitation Society. Ann Fr Anesth Reanim. 2008; 27: 541-551.
- Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators; ANZICS Clinical Trials Group. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012; 186 :724-731.
- Shehabi Y, Bellomo R, Kadiman S, Ti LK, Howe B, Reade MC, et al. Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Sedation Intensity in the First 48 Hours of Mechanical Ventilation and 180- Day Mortality: A Multinational Prospective Longitudinal Cohort Study. Crit Care Med. 2018; 46: 850-859.
- Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health- System Pharmacists (ASHP), American College of Chest Physicians. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30: 119-413.
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166: 1338- 1344.
- Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003; 289: 2983- 2991.
- Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001; 29: 2258-2263.
- Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998; 114: 541-548.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342: 1471-1417.
- Mehta S, Burry L, Martinez-Motta JC, Stewart TE, Hallett D, McDonald E, et al. Canadian Critical Care Trials Group. A randomized trial of daily awakening in critically ill patients managed with sedation protocol: a pilot trial. Crit Care Med. 2008; 36: 2092-2099.
- Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, et al. SLEAP Investigators; Canadian Critical Care Trials Groupe. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012; 308: 1985- 1992.
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006; 354: 449-461.
- Le gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270: 2957-2963.
- Rello J, Paiva JA, Baraibar J, Barcenilla F, Bodi M, Castander D, et al. International Conference for the Development of Consensus on the Diagnosis and treatment of Ventilator-associated Pneumonia. Chest. 2001; 120: 955- 970.
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001; 29: 1370-1379.
- Vincent JL, De Mendoça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on «sepsis-related problems» of the European Society of Intensive Care Medicine. Crit Care Med. 1998; 26: 1793-1800.
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999; 27: 2609-2615.
- Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. Qual Saf Health Care. 2004; 13 :203-205.
- De Jonghe B, Bastuji-Garin S, Fangio P, Lacherade JC, Jabot J, Appéré-De- Vecchi C, et al. Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med. 2005; 33: 120-127.
- Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004; 32: 1272-1276.
- Arias-Rivera S, Sanchez-Sanchez Mdel M, Santos-Dias R, Gallardo-Murillo J, Sánchez-Izquierdo R, Frutos-Vivar F, Ferguson ND, et al. Effect of a nursing-implemented sedation protocol on weaning outcome. Crit care med. 2008; 36: 2054-2060.
- Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Med. 2006; 32: 1506-1514.
- Frawley A, Hickey J, Weaver C, Williams JP, Szakmany T. Introducing a new sedation policy in a large district general hospital: before and after cohort analysis. Anaesthesiol Intensive Ther. 2019.
- Balas MC, Pun BT, Pasero C, Engel HJ, Perme C, Esbrook CL, et al. Common Challenges to Effective ABCDEF Bundle Implementation: The ICU Liberation Campaign Experience. Crit Care Nurse. 2019; 39: 46-60.
- Hsieh SJ, Otusanya O, Gershengorn HB, Hope AA, Dayton C, Levi D, et al. Staged Implementation of Awakening and Breathing, Coordination, Delirium Monitoring and Management, and Early Mobilization Bundle Improves Patient Outcomes and Reduces Hospital Costs. Crit Care Med. 2019; 47: 885-893.
- Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2019; 47: 3-14.
- Langer M, Mosconi P, Cigada M, Mandelli M. Long-term respiratory support and risk of pneumonia in critically ill patients. Intensive Care Unit Group of Infection Control. Am Rev Respir Dis. 1989; 140: 302-305.
- Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015; 41: 34-48.
- Pottier V, Daubin C, Lerolle N, Gaillard C, Viquesnel G, Plaud B, et al. Charbonneau P. Overview of adverse events related to invasive procedures in the intensive care unit. Am J Infect Control. 2012; 40: 241-246.
- Quenot JP, Ladoire S, Devoucoux F, Doise JM, Cailliod R, Cunin N, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007; 35: 2031-2036.
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41: 263-306.
- Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007; 33: 66-73.
- Barnes-Daly MA, Phillips G, Ely EW. Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit Care Med. 2017; 45: 171-178.
- Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008; 36: 427-433.
- Dodek P, Chanques G, Brown G, Norena M, Grubisic M, Wong H, Jaber S. Role of organisational structure in implementation of sedation protocols: a comparison of Canadian and French ICUs. BMJ Qual Saf. 2012; 21: 715-721
- Pun BT, Gordon SM, Peterson JF, Shintani AK, Jackson JC, Foss J, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med. 2005; 33: 1199-1205.
- Lucidarme O, Seguin A, Daubin C, Ramakers M, Terzi N, et al. Nicotine withdrawal and agitation in ventilated critically ill patients. Crit Care. 2010; 14: 58.
- Hugues CG, Girard TD, Pandharipande PP. Daily sedation interruption versus targeted light sedation strategies in ICU patients. Crit Care Med. 2013; 41: 40-45.
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients. JAMA. 2009; 301: 489-499.
- Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and metaanalysis of randomized trials. Crit Care Med. 2013; 41: 30-38.
- Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Executive Summary: Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018; 46: 1532-1548
- Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Posttraumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007; 11: 27.
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, et al. Delirium as a prédictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291: 1753-1762.