
Research Article
Austin Crit Care J. 2022; 9(1): 1041.
Relationship of Visceral Adiposity Index with New-Onset Hyperuricemia in Hypertensive Patients
Zhang S1,2#, Li Q1,2#, Li R1,2, Zhang Y3, Li H3, He P3, Liu C1,2, Li J3, Zhang Y4, Huo Y5, Qin X1,2,3*, Xu X1* and Wang B1,2,6*
1Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, China
2Institute of Biomedicine, Anhui Medical University, Hefei, China
3Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center for Kidney Disease; State Key Laboratory of Organ Failure Research; Guangdong Provincial Institute of Nephrology, Guangdong Provincial Key Laboratory of Renal Failure Research, Guangzhou Regenerative Medicine and Health Guangdong Laboratory, Guangzhou, China
4Department of Cardiology, Peking University First Hospital, Beijing, China
5Beijing Advanced Innovation Center for Food Nutrition and Human Health, the Key Laboratory for Functional Dairy, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing, China
6Shenzhen Evergreen Medical Institute, Shenzhen, China
#Contributed Equally to this manuscript
*Corresponding author: Xianhui Qin, Institute of Biomedicine, Anhui Medical University, Hefei 230032, China
Xiping Xu, Institute of Biomedicine, Anhui Medical University, Hefei, China
Binyan Wang, Institute of Biomedicine, Anhui Medical University, Hefei 230032, China
Received: January 24, 2022; Accepted: February 21, 2022; Published: February 28, 2022
Abstract
Background: Visceral adiposity index is a new type of indicator that accurately reflects distribution and function of visceral fat. The relation between VAI and new-onset hyperuricemia remains largely understudied.
Purpose: This study sought to further investigate the prospective association between VAI and the risk of hyperuricemia by examining possible effect modifies in hypertensive patients.
Methods: We enrolled 10,513 hypertensive patients with normal uric acid (UA) concentrations (<357 μmol/L (6 mg/dL)) who participated the UA Sub-study of the China Stroke Primary Prevention Trial (CSPPT). Our primary outcome was new-onset hyperuricemia, which was defined as a UA concentration ≥417 μmol/L (7 mg/dL) in men or ≥357 μmol/L (6 mg/dL) in women at the exit visit.
Results: Over a median follow-up of 4.4 years, 1,642 (15.6%) participants developed new-onset hyperuricemia. When VAI was assessed as quartiles, a significantly higher risk of new-onset hyperuricemia was found in participants in quartile 4 (≥2.98; odds ratio, 1.17; 95% CI: 1.01-1.36) compared with those in quartile 1-3 (<2.98). Furthermore, we discovered that the positive relation was independent of abnormal VAI components or numbers of abnormal VAI components (all P-interactions > 0.05).
Conclusion: There was a positive relationship between baseline VAI and the risk of new-onset hyperuricemia in a sample of Chinese hypertensive individuals.
Keywords: Visceral adiposity index; Uric acid; New-onset hyperuricemia
Introduction
In recent years, an increasing trend in the prevalence of hyperuricemia has been observed in epidemiological studies [1,2]. Patients with hyperuricemia sustained increasing risk of gout, cardiovascular diseases (CVD), diabetes and chronic kidney disease (CKD) [3-6]. Hence, the discovery of more modifiable risk factors related to hyperuricemia is important for preventing hyperuricemia and reducing the risk of its related diseases.
Obesity is a major global health challenge and is also an important risk factor for cancer, diabetes and CKD [7-9]. Several studies have suggested that it is not the extent of obesity but the distribution of adiposity tissue that plays a decisive role in the impact of obesity on these diseases [10,11]. In addition, previous study has found that the an increase in visceral adiposity is associated with the higher risk prevalence of hyperuricemia [12].
There are several traditional methods like body mass index (BMI), waist-to-height ratio, waist circumference (WC), waist-tohip ratio, but none of these can measure visceral adiposity accurately [13]. The visceral adiposity index (VAI) is as accurate as magnetic resonance imaging (the gold standard method) in measuring visceral adiposity [14], and therefore, can be used as a valuable indicator of lipid accumulation and visceral adipose function for its convenience and accuracy. However, several cross-sectional studies [15,16] and only one prospective study [17] have evaluated the association between VAI and hyperuricemia, and reported inconsistent findings. Furthermore, few studies have been conducted in hypertensive population, who are proved to be at high risk for hyperuricemia [18].
To address the aforementioned gaps in the existing literature, we aimed to further investigate the prospective association between VAI and the risk of hyperuricemia by examining possible effect modifies in hypertensive patients who joined the UA Sub-study of the China Stroke Primary Prevention Trial (CSPPT) [19].
Methods
Study design and population
The study procedures have been described in previous studies [19-23], and are therefore only briefly explained here. The CSPPT was a multi-community, randomized, double-blind controlled trial with 20,702 hypertensive adults in 32 communities in Jiangsu and Anhui provinces of China, which was conducted from May 19, 2008 to August 24, 2013.
The UA sub-study of the CSPPT enrolled 15,364 eligible participants with complete data on UA and without the usage of UAlowering drugs at baseline from 20 communities in Jiangsu province. The current study is a post-hoc analysis of the UA Sub-study. The flow of the participants is presented in Supplemental Figure 1.
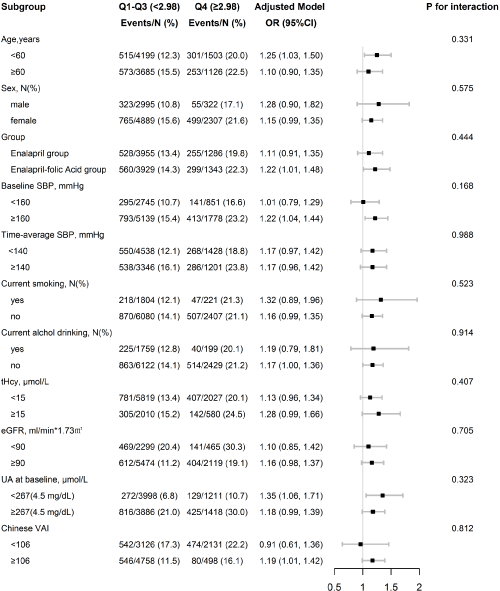
Figure 1: Stratified analysis of the impact of VAI on new-onset hyperuricemia in various subgroups*.
*Adjusted for treatment group, age, sex, uric acid (UA), fasting glucose, total cholesterol, total homocysteine (tHcy), estimated glomerular filtration rate (eGFR),
smoking and drinking status, use of antihypertensive drugs, body mass index (BMI), waist circumference (WC), TG/HDL-C ratio, SBP at baseline and timeaveraged
on treatment SBP during treatment.
Intervention and follow-up
Eligible participants were randomly assigned, in a 1:1 ratio, to one of two treatment groups: a daily oral dose of one tablet containing 10mg enalapril and 0.8mg folic acid (single pill combination, the enalapril-folic acid group), or a daily oral dose of one tablet containing 10mg enalapril only (the enalapril group).
Participants were scheduled for followed up every three months. At each follow-up visit, BP was measured; study drug adherence, concomitant medication use, adverse events and possible endpoint events were documented by trained research staff and physicians. During the trial period, if blood pressure (BP) was not adequately controlled, other classes of anti-hypertensive medications, mostly nitrendipine or hydrochlorothiazide, could be prescribed concomitantly. At the exit visit, final blood samples were collected and assessed.
Anthropometric measurements
Height was measured without shoes to the nearest 0.1 cm on a portable stadiometer. Weight was measured in light indoor clothing without shoes to the nearest 0.1 kg. BMI was calculated as weight (kilograms)/height (meters) squared. WC was measured as the minimum circumference between the inferior margin of the ribcage and the crest of the ileum [24-26].
Laboratory assays
Serum concentrations of UA, fasting glucose, total homocysteine (tHcy) and lipids were measured with automatic analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Guangzhou, China. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [27].
Outcomes
The primary outcome was new-onset hyperuricemia in hypertensive participants with normal UA concentrations (<357μmol/L (6mg/dL)) at baseline. Hyperuricemia was defined as a UA concentration ≥417μmol/L (7mg/dL) in men or ≥357μmol/L (6mg/dL) in women [19,28,29].
The secondary outcome was change in UA concentrations, defined as UA concentrations at the exit visit minus that at baseline.
Major definitions
The VAI, a reliable index based on WC, BMI, TG and HDL-C, was calculated using the following formulas 14:
Abnormal VAI components were identified as the presence of any of the following one components 30, 31: (1) higher BMI (BMI ≥ 28 kg/m²); (2) higher WC (WC ≥ 85 cm for females and ≥ 90 cm for males); (3) elevated TG (≥ 1.7 mmol/L); (4) lower HDL-C (<1.04 mmol/L for males and <1.30 mmol/L for females).
The Chinese VAI (CVAI) was estimated as follows: Males: -267.93 + 0.68*age + 0.03*BMI + 4.00*WC + 22.00*Log10TG- 16.32*HDL-C; Females: -187.32 + 1.71*age + 4.23*BMI + 1.12*WC + 39.76*Log10TG - 11.66*HDL-C 32.
Statistical analyses
Baseline characteristics of study population are expressed as mean ± standard deviation (SDs) for continuous variables and as frequencies and percentages for categorical variables, respectively. To assess whether there were significant differences in baseline levels of participants by VAI quartiles, we used analysis of variance tests for continuous variables or chi-square tests for categorical variables.
The relationship of baseline VAI with primary and secondary outcomes were examined using multivariable logistic regression models and generalized linear regression models, respectively, without and with adjustment for covariates including age, sex, treatment group, UA, fasting glucose, total cholesterol, total homocysteine (tHcy), estimated glomerular filtration rate (eGFR), SBP, smoking and drinking status, use of antihypertensive drugs, as well as time-averaged SBP during treatment period in Model 2; and all the variables in Model 2 plus BMI, WC, TG/HDL-C ratio at baseline in Model 3. As additional exploratory analysis, possible modifications on the association between VAI and new-onset hyperuricemia were also evaluated by stratified analyses and interaction testing.
two-tailed P<0.05 was considered to be statistically significant in all analyses. Statistical analyses were performed using R software, version 3.6.3 (http://www. R-project.org/).
Results
Study participants and baseline characteristics
A total of the 10,513 participants with complete data on baseline VAI and exit UA, who were not using UA lowering drugs during the follow up period, as well as whose baseline UA levels were <357μmol/L (6mg/dL) in the UA Sub-study of CSPPT (Supplemental Figure 1) were included in the final analysis.
Baseline characteristics of the study participants by VAI quartiles are shown in Table 1. The mean age of the participants was 59.4 ± 7.4 years; 3317 (31.6%) were men. The median baseline VAI was 1.87 (interquartile range: 1.18-2.98). Participants with higher VAI were more likely to be females and younger; tend to be current smoker and alcohol drinker; had higher BMI, WC, fasting glucose, TG, TC and eGFR levels, as well as higher time-averaged SBP during the treatment period; lower HDL-C, tHcy, folate levels; higher frequency usage of antihypertensive drugs, glucose-lowering drugs; and higher prevalence of self-reported diabetes and hyperlipidemia at baseline.
Variables
Visceral adiposity index
P value
Q1 (<1.18)
Q2 (1.18-<1.87)
Q3 (1.87-<2.98)
Q4 (=2.98)
N
2628
2628
2628
2629
Age, y
60.0 ± 7.6
59.3 ± 7.5
59.3 ± 7.4
59.1 ± 7.2
<0.001
Male, n (%)
1637 (62.3)
837 (31.8)
521 (19.8)
322 (12.2)
<0.001
Waist circumference, cm
79.8 ± 8.4
83.8 ± 9.1
86.8 ± 8.9
89.2 ± 8.5
<0.001
Body mass index, kg/m2
23.7 ± 3.0
25.1 ± 3.3
26.3 ± 3.4
27.0 ± 3.4
<0.001
Visceral adiposity index
0.8 ± 0.2
1.5 ± 0.2
2.4 ± 0.3
4.8 ± 3.9
<0.001
Current smoking
971 (36.9)
512 (19.5)
321 (12.2)
221 (8.4)
<0.001
Current alcohol drinking
1015 (38.6)
455 (17.3)
289 (11.0)
199 (7.6)
<0.001
Self-reported diabetes
48 (1.8)
82 (3.1)
101 (3.8)
169 (6.4)
<0.001
Self-reported hyperlipidemia
35 (1.3)
47 (1.8)
87 (3.3)
100 (3.8)
<0.001
BP, mmHg
Baseline SBP
168.8 ± 20.6
168.4 ± 20.9
169.1 ± 21.3
169.5 ± 20.3
0.32
Baseline DBP
95.2 ± 12.1
94.6 ± 11.9
94.9 ± 11.6
95.1 ± 11.6
0.299
SBP during treatment period
139.1 ± 10.8
138.9 ± 10.7
139.3 ± 10.9
139.8 ± 10.9
0.014
DBP during treatment period
83.3 ± 7.2
83.1 ± 7.2
83.1 ± 7.0
83.4 ± 7.0
0.33
Laboratory results
Total cholesterol, mmol/L
5.6 ± 1.1
5.7 ± 1.1
5.8 ± 1.2
5.8 ± 1.3
<0.001
Folate, ng/mL
7.7 ± 3.3
7.8 ± 3.2
7.9 ± 3.4
7.5 ± 3.0
<0.001
Fasting glucose, mmol/L
5.8 ± 1.4
6.0 ± 1.7
6.1 ± 1.9
6.4 ± 2.3
<0.001
Triglycerides, mmol/L
0.9 ± 0.2
1.3 ± 0.3
1.7 ± 0.4
2.7 ± 2.1
<0.001
HDL-C, mmol/L
1.6 ± 0.4
1.4 ± 0.3
1.3 ± 0.2
1.1 ± 0.2
<0.001
TG/HDL-C ratio
0.8 ± 0.4
1.2 ± 0.6
1.5 ± 1.0
2.1 ± 2.2
<0.001
Total homocysteine, μmol/L
14.7 ± 8.9
13.9 ± 7.9
14.0 ± 8.6
13.7 ± 8.0
<0.001
eGFR, mL/min/1.73m2
93.6 ± 11.1
93.6 ± 11.7
95.1 ± 11.6
98.6 ± 12.0
<0.001
UA, μmol/L
266.6 ± 52.3
261.3 ± 52.7
263.4 ± 51.3
268.4 ± 51.4
<0.001
Medication use, n (%)
Antihypertensive drugs
1007 (38.3)
1214 (46.2)
1328 (50.5)
1438 (54.7)
<0.001
Glucose-lowering drugs
30 (1.1)
35 (1.3)
55 (2.1)
72 (2.7)
<0.001
Antiplatelet drugs
93 (3.5)
85 (3.2)
89 (3.4)
94 (3.6)
0.901
Variables are presented as Mean ± SD or n (%).
Abbreviations: BP: Blood Pressure; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure; eGFR: estimated Glomerular Filtration Rate; UA: Uric Acid; TG: Triglycerides; HDL-C: High Density Lipoprotein Cholesterol.
Table 1: Characteristics of study participants by quartiles of visceral adiposity index.
Additionally, participants with higher baseline VAI had a higher frequency in the use of glucose-lowering drugs and antiplatelet drugs during the treatment period (Supplemental Table 1).
Relationship of VAI level with study outcome
During a median follow-up duration of 4.4 years, a total of 1663 (15.7%) participants developed new-onset hyperuricemia.
Subsequently, participants were stratified into quartiles according to VAI (quartile 1, lowest; quartile 4, highest). And when we used the lowest quartile 1 (Q1: <1.18) as a reference, the ORs for the second (Q2: 1.18-1.87), third (Q3: 1.87-2.98), and fourth quartiles (Q4: ≥2.98) of (95%CI) for participants were 1.10 (0.91-1.32), 1.18 (0.97- 1.43), and 1.33 (1.06-1.65), respectively (P for trend = 0.010).
Consistently, higher risk of new-onset hyperuricemia (OR, 1.27; 95% CI: 1.12-1.45) was discovered in participants in quartile 4 of VAI level (≥ 2.98) compared with those in quartile 1-3 (< 2.98). Moreover, further adjustment for BMI, WC, TG/HDL-C ratio did not substantially affect the relationship of higher VAI level (≥ 2.98) with new-onset hyperuricemia (OR, 1.17; 95% CI: 1.01-1.36) (Table 2). The similar results were found for the secondary outcome (Table 3). The similar results were also observed when UA concentrations <417 μmol/L for male and <357 μmol/L for female at baseline were included the analysis (Supplemental Tables 2 and 3).
Visceral adiposity index
Events/N (%)
Model 1
Model 2
Model 3
OR (95%CI)
P value
OR (95%CI)
P value
OR (95%CI)
P value
Quartiles
Q1 (<1.18)
276/2628 (10.5)
ref.
ref.
ref.
Q2 (1.18-<1.87)
365/2628 (13.9)
1.12 (0.94, 1.34)
0.205
1.16 (0.97, 1.40)
0.107
1.10 (0.91, 1.32)
0.327
Q3 (1.87-<2.98)
447/2628 (17.0)
1.25 (1.04, 1.49)
0.015
1.30 (1.08, 1.57)
0.005
1.18 (0.97, 1.43)
0.106
Q4 (=2.98)
554/2629 (21.1)
1.42 (1.19, 1.69)
<0.001
1.52 (1.26, 1.83)
<0.001
1.33 (1.06, 1.65)
0.012
P for trend
<0.001
<0.001
0.01
Categories
Q1-Q3 (<2.98)
1088/7884 (13.8)
ref.
ref.
ref.
Q4 (=2.98)
554/2629 (21.1)
1.23 (1.09, 1.40)
<0.001
1.27 (1.12, 1.45)
<0.001
1.17 (1.01, 1.36)
0.039
Model 1: Adjusted for age, sex and uric acid (UA) at baseline. Model 2: Adjusted for age, sex, UA, fasting glucose, total cholesterol (TC), total homocysteine (tHcy), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP), smoking and drinking status, use of antihypertensive drugs at baseline, treatment group, and mean SBP during the treatment period.
Model 3: Adjusted for the variables in model 2 plus body mass index (BMI), waist circumference (WC), TG/HDL-C ratio.
Table 2: The association between baseline visceral adiposity index and new-onset hyperuricemia.
Visceral adiposity index
UA change, μmol/L
Model 1
Model 2
Model 3
Mean ± SD
β (95%CI)
P value
β (95%CI)
P value
β (95%CI)
P value
Quartiles
Q1 (<1.18)
40.7 ± 64.4
ref.
ref.
ref.
Q2 (1.18-<1.87)
41.8 ± 62.8
5.53 (2.02, 9.04)
0.002
6.65 (3.12, 10.17)
<0.001
4.47 (0.86, 8.08)
0.015
Q3 (1.87-<2.98)
42.1 ± 65.5
8.81 (5.19, 12.43)
<0.001
10.40 (6.72, 14.08)
<0.001
6.25 (2.28, 10.21)
0.002
Q4 (=2.98)
42.2 ± 66.9
12.12 (8.39, 15.84)
<0.001
14.63 (10.72, 18.54)
<0.001
8.32 (3.49, 13.15)
<0.001
P for trend
<0.001
<0.001
<0.001
Categories
Q1 (<1.18)
40.7 ± 64.4
ref.
ref.
ref.
Q2-Q4 (=1.18)
42.1 ± 65.1
6.64 (3.76, 9.52)
<0.001
9.89 (6.83, 12.95)
<0.001
5.39 (2.08, 8.71)
0.001
*Change in uric acid concentrations was defined as the uric acid concentration at the exit visit minus that at baseline;
Model 1: Adjusted for age, sex, and uric acid (UA) at baseline.
Model 2: Adjusted for age, sex, UA, fasting glucose, total cholesterol (TC), total homocysteine (tHcy), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP), smoking and drinking status, use of antihypertensive drugs at baseline, treatment group, and mean SBP during the treatment period.
Model 3: Adjusted for the variables in model 2 plus body mass index (BMI), waist circumference (WC), TG/HDL-C ratio.
Table 3: The association between baseline visceral adiposity index and change in uric acid concentrations.
Of note, further adjustment for the use of glucose-lowering drugs and antiplatelet drugs during the treatment period also did not significantly change the results (Supplemental Table 4).
Subgroups†
Q1-Q3 (<2.98)
Events/N (%)Q4 (=2.98)
Events/N (%)Adjusted Model*
OR (95% CI)P for interaction
Higher BMI†
0.658
yes
285/1429 (19.9)
232/931 (24.9)
1.12 (0.89, 1.40)
No
803/6455 (12.4)
322/1698 (19.0)
1.19 (1.00, 1.42)
Higher WC†
0.864
Yes
671/4042 (16.6)
494/2260 (21.9)
1.16 (0.99, 1.35)
No
417/3842 (10.9)
60/369 (16.3)
1.12 (0.81, 1.56)
Elevated TG†
0.66
Yes
223/1435 (15.5)
518/2445 (21.2)
1.28 (1.05, 1.56)
No
865/6449 (13.4)
36/184 (19.6)
1.16 (0.77, 1.74)
Lower HDL-C†
0.877
Yes
329/2158 (15.2)
459/2192 (20.9)
1.10 (0.92, 1.33)
No
759/5726 (13.3)
95/437 (21.7)
1.13 (0.94, 1.35)
Number of abnormal VAI components†
0.932
=1
578/5053 (11.4)
14/89 (15.7)
1.08 (0.58, 2.03)
2
332/1945 (17.1)
101/587 (17.2)
1.06 (0.80, 1.39)
=3
178/886 (20.1)
439/1953 (22.5)
1.23 (0.98, 1.53)
*If not stratified, adjusted for age, sex, UA, fasting glucose, total cholesterol (TC), total
homocysteine (tHcy), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP),
smoking and drinking status, use of antihypertensive drugs at baseline, treatment group, and
mean SBP during the treatment period, body mass index (BMI) ,waist circumference (WC),
TG/HDL-C ratio.
† Higher body mass index was defined as BMI =28 kg/m2; higher waist circumference was
defined as WC =80 in females or WC =90 in males; elevated triglycerides were defined as TG
=1.7 mmol/L; lower high-density lipoprotein was defined as HDL-C <1.30 mmol/L in females
or HDL-C <1.04 mmol/L in males; number of abnormal VAI components was defined as
number of high body mass index, higher circumference, high triglycerides or low high density
lipoprotein cholesterol.
Table 4: Stratified analysis of the impact of VAI on new-onset hyperuricemia by VAI components.
Subgroup analysis by single VAI components
Subgroup analyses were conducted to examine whether the positive association confounded by single VAI components (Table 4). Then, we found that the positive relation was consistent in participants with or without higher BMI, higher WC, elevated TG and decreased HDL-C and there were no significant interactions between VAI and single VAI components or abnormal numbers of VAI components (≤1, 2, and ≥3) on new-onset hyperuricemia (all P for interactions > 0.05) (Table 4).
Stratified analyses by other potential effect modifiers
Stratified analyses were performed using several identified risk factors to further assess the relationship of VAI (Figure 1) with the risk of new-onset hyperuricemia. None of the variables, including age (<60 vs. ≥60 years), sex (male vs. female), treatment group (enalapril group vs. enalapril-folic acid group), SBP (<160 vs. ≥160 mmHg), tHcy (<15 vs. ≥15 μmol/L), eGFR (<90 vs. <90 mL/min/1.73m2), current smoking status (yes vs. no), UA (<267 vs. <267 μmol), Chinese VAI (median, <106 vs. <106), current alcohol drinking (yes vs. no) at baseline and time-averaged SBP (<140 vs. <140 mmHg) during treatment, significantly modified the association between VAI and the risk of new-onset hyperuricemia in hypertensive patients (all P for interactions > 0.05) (Figure 1).
Discussion
The current study demonstrates that baseline VAI was positively associated with the risk of new-onset hyperuricemia during a median follow-up of 4.4 years in Chinese hypertensive patients. The positive relationship was independent of VAI components (BMI, WC, TG and HDL-C).
To our knowledge, inconsistent results have been reported regarding the relationship of VAI with hyperuricemia in several cross-sectional studies. Dong et al. found that there was a significant positive relation of the VAI with hyperuricemia in the Chinese populations [15]. In contrast, Liu et al. reported that VAI was not associated with the prevalence of hyperuricemia among Chinese population [16]. Only a prospective cohort study of 1936 healthy workers aged 6 to 82 years in Mexico had been conducted to examine the association between VAI and the risk of hyperuricemia, and showed that individuals in the highest VAI quartile had higher odds for hyperuricemia compared with individuals from the lowest quartile [17]. However, this study only adjusted for age, alcohol consumption, smoking status and physical activity, and did not consider the effect of other important confounders. As such, the study could not provide an accurate measurement for the independent relation of VAI with hyperuricemia.
Of note, a CVAI was also created in a previous study in Chinese [32]. As were reported, VAI was significantly correlated with visceral adipose volume measured by MRI, while CVAI was well associated with visceral adipose area measured by CT [32,33]. Base on a large sample of individuals, comprehensive adjustments of major traditional risk factors for hyperuricemia and the components of VAI and CVAI, we discovered that VAI levels was prospective positively associated with the risk of new hyperuricemia in patients with hypertension. Moreover, the positive relationship persisted in those with or without higher BMI, higher WC, elevated TG, decreased HDL-C and elevated CVAI. Therefore, our results suggest that VAI can be used as both an indicator of adipose tissue distribution and a functional indicator to assess the risk of hyperuricemia, independent of BMI, WC, TG, HDL-C or even CVAI.
The potential mechanisms by which higher VAI increases the risk of hyperuricemia is unclear, but it is biologically plausible. Many studies have shown that pathological visceral adipose tissue is considered to be metabolically active. In this condition, adipose tissue abnormally releases cytokines such as leptin and adiponectin [15,34,35]. Abnormal release of these adipocytokines may cause insulin resistance which may enhance renal proximal tubular reabsorption of UA with a subsequent increase in serum UA levels [36,37]. The increase in visceral adiposity accumulation provides excess free fatty acids - products of fatty breakdown - that may be associated with purine synthesis, which may accelerate UA production [38]. Third, the visceral fat volume may be more accurate in reflecting the visceral fat accumulation. Further research is required to identify mechanisms underlying an association between VAI and new-onset hyperuricemia.
Indeed, several limitations in our study that merit emphasis. First, in this post-hoc analysis, many covariates had been adjusted in the regression models; however, residual confounding from unmeasured or unrecorded factors may work. Second, this post-hoc analysis focused on Chinese hypertensive participates, so the generalizability of these findings to other types of populations remains to be unknown. Third, serum UA levels were measured only at baseline and exit visits. More frequent measurements of serum uric acid levels are needed to more accurately assess the relationship between VAI and new-onset hyperuricemia in the duration. Although there are many limitations, our results serve as the basis for future relevant randomized trials.
Conclusions
In conclusion, our results suggest that higher VAI is significantly associated with increased risk of new-onset hyperuricemia in Chinese hypertensive patients, independent of single VAI components. VAI can be easily measured and applied to clinical practice. Thus, VAI has important implications for primary prevention and early detection of new-onset hyperuricemia.
Declaration
Contributors: Study conception and design: Binyan Wang, Xianhui Qin, Xiping Xu, Shaojie Zhang; Acquisition of data: Binyan Wang, Xianhui Qin; Analysis and interpretation of data: Shaojie Zhang, Xianhui Qin; Drafting of the manuscript: Shaojie Zhang, Xianhui Qin; All authors critically revised the manuscript, gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding: The study was supported by funding from the following: the National Key Research and Development Program (2016YFE0205400, 2018ZX09739, 2018ZX093 01034003); the National Natural Science Foundation of China (81730019, 81973133; the Science and Technology Planning Project of Guangzhou, China (201707020010); the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155 639040, GJHS20170314114526143); the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390).
Competing interests: Dr. Xi-Ping Xu reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, 2018ZX093010 34003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143), and the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390). Dr. Xian- Hui Qin reports grants from the National Natural Science Foundation of China (81730019, 81973133).
Ethics approval: The present study were approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263).
References
- Liu R, Han C, Wu D, et al. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015; 2015: 762820.
- Kumar AUA, Browne L, Li X, et al. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: A cohort study. PLoS One. 2018; 13: e0198197.
- Dalbeth N, Merriman TR, Stamp LK. Gout. The Lancet. 2016; 388: 2039-2052.
- Braga F, Pasqualetti S, Ferraro S, et al. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016; 54: 7-15.
- Zhou C, Liu M, Zhang Z, et al. Positive association of serum uric acid with new-onset diabetes in Chinese women with hypertension in a retrospective analysis of the China Stroke Primary Prevention Trial. Diabetes Obes Metab. 2020; 22: 1598-1606.
- Maruhashi T, Hisatome I, Kihara Y, et al. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis. 2018; 278: 226-231.
- Himbert C, Delphan M, Scherer D, et al. Signals from the Adipose Microenvironment and the Obesity-Cancer Link-A Systematic Review. Cancer Prev Res (Phila). 2017; 10: 494-506.
- Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015; 314: 1052-1062.
- Liu M, Zhang Z, Zhou C, et al. Relationship of Body Mass Index and Waist Circumference with Risk of New-Onset Proteinuria in Hypertensive Patients. J Clin Endocrinol Metab. 2020; 105.
- Pischon T, Boeing H, Hoffmann K, et al. General and Abdominal Adiposity and Risk of Death in Europe. N Engl J Med. 2008; 13: 2105-2120.
- Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest. 2013; 36: 537-543.
- Yamada A, Sato KK, Kinuhata S, et al. Association of Visceral Fat and Liver Fat With Hyperuricemia. Arthritis Care Res (Hoboken). 2016; 68: 553-561.
- Biswas E, Choudhury AK, Amin MR, et al. Visceral Adiposity Index Score is the Better Predictor of Clinical and Coronary Angiographic Severity Assessment than Other Adiposity Indices in Patients with Acute Coronary Syndrome. Mymensingh Med J. 2019; 28: 382-388.
- Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. 2014; 2014: 730827.
- Dong H, Xu Y, Zhang X, et al. Visceral adiposity index is strongly associated with hyperuricemia independently of metabolic health and obesity phenotypes. Sci Rep. 2017; 7: 8822.
- Liu XZ, Li HH, Huang S, et al. Association between hyperuricemia and nontraditional adiposity indices. Clin Rheumatol. 2019; 38: 1055-1062.
- Rivera-Paredez B, Macias-Kauffer L, Fernandez-Lopez JC, et al. Influence of Genetic and Non-Genetic Risk Factors for Serum Uric Acid Levels and Hyperuricemia in Mexicans. Nutrients. 2019; 11.
- Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood Pressure control rate and Cardiovascular Risk profile study. J Hypertens. 2019; 37: 380-388.
- Qin X, Li Y, He M, et al. Folic acid therapy reduces serum uric acid in hypertensive patients: a substudy of the China Stroke Primary Prevention Trial (CSPPT). Am J Clin Nutr. 2017; 105: 882-889.
- Qin X, Spence JD, Li J, et al. Interaction of serum vitamin B12 and folate with MTHFR genotypes on risk of ischemic stroke. Neurology. 2020; 94: e1126-e1136.
- Qin X, Shen L, Zhang R, et al. Effect of folic acid supplementation on cancer risk among adults with hypertension in China: A randomized clinical trial. Int J Cancer. 2017; 141: 837-847.
- Qin X, Li J, Zhang Y, et al. Effect of folic acid supplementation on risk of newonset diabetes in adults with hypertension in China: Findings from the China Stroke Primary Prevention Trial (CSPPT). J Diabetes. 2016; 8: 286-294.
- Wang B, Wu H, Li Y, et al. Effect of long-term low-dose folic acid supplementation on degree of total homocysteine-lowering: major effect modifiers. Br J Nutr. 2018; 120: 1122-1130.
- He M, Qin X, Cui Y, et al. Prevalence of unrecognized lower extremity peripheral arterial disease and the associated factors in Chinese hypertensive adults. Am J Cardiol. 2012; 110: 1692-1698.
- Qin X, Li J, Zhang Y, et al. Prevalence and associated factors of diabetes and impaired fasting glucose in Chinese hypertensive adults aged 45 to 75 years. PLoS One. 2012; 7: e42538.
- Qin X, Zhang Y, Cai Y, et al. Prevalence of obesity, abdominal obesity and associated factors in hypertensive adults aged 45-75 years. Clin Nutr. 2013; 32: 361-367.
- Stockman JA. A New Equation to Estimate Glomerular Filtration Rate. Yearbook of Pediatrics. 2011; 2011: 193-194.
- Cao J, Zhang J, Li Q, et al. Serum Phosphate and the Risk of New-Onset Hyperuricemia in Hypertensive Patients. Hypertension. 2019; 74: 102-110.
- Feig DI KD, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008; 359: 1811-1821.
- Zhang L, Wang Z, Wang X, et al. Prevalence of Abdominal Obesity in China: Results from a Cross-Sectional Study of Nearly Half a Million Participants. Obesity (Silver Spring). 2019; 27: 1898-1905.
- Expert Panel on Detection E and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285: 2486-2497.
- Xia MF, Chen Y, Lin HD, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016; 6: 38214.
- Xia MF, Lin HD, Chen LY, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metab Res Rev. 2018; 34: e3048.
- Goodpaster BH KS, Resnick H, Kelley DE, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003; 26: 372-379.
- Kanaya AM HT, Goodpaster BH, Tylavsky F, et al. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004; 27: 1375-1380.
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010; 11: 11-18.
- Facchini F CY, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991; 266: 3008-3011.
- Stepien M, Stepien A, Banach M, et al. New obesity indices and adipokines in normotensive patients and patients with hypertension: comparative pilot analysis. Angiology. 2014; 65: 333-342.