
Case Report
Austin Crit Care J. 2022; 9(1): 1042.
Extremely Severe Hypoglycemia in a Patient Who Experienced Spontaneous Rupture of a Hepatocellular Carcinoma
Honda A1, Masada T2, Morita M3, Kono F4, Imashuku S5*
1Department of Internal Medicine, Uji-Tokushukai Medical Center, Uji, Kyoto, Japan
2Department of Radiology, Uji-Tokushukai Medical Center, Uji, Kyoto, Japan
3Department of Internal Medicine 1, Shimane University Faculty of Medicine, Izumo, Shimane, Japan
4Division of Diagnostic Pathology, Uji-Tokushukai Medical Center, Uji, Kyoto, Japan
5Department of Laboratory Medicine, Uji-Tokushukai Medical Center, Uji, Kyoto, Japan
*Corresponding author: Shinsaku Imashuku, Department of Laboratory Medicine, Uji-Tokushukai Medical Center, Uji, Kyoto, 611-0041, Japan
Received: February 25, 2022; Accepted: March 15, 2022; Published: March 22, 2022
Abstract
An 85-year-old woman was brought to the emergency room because of shock and coma. She was found to have extreme hypoglycemia (blood glucose <10 mg/dL), associated with the spontaneous rupture of an endstage Hepatocellular Carcinoma (HCC). Because the patient had declined any treatment for her HCC during the preceding year, no specific treatment was instituted for the ruptured HCC. Intravenous infusion of high-glucose fluid normalized her blood glucose concentration within 4 hours, but she died further 6 hours later. At autopsy, neither insulinoma nor a malignancy other than the HCC was identified. Her liver weighed 1,290 g and was filled with tumor masses. The HCC cells were negative for insulin-like growth factor 2 expression on immunohistochemistry, indicating that the extreme hypoglycemia was not a “Non-Islet Cell Tumor Hypoglycemia (NICHT)”.
Keywords: Hypoglycemia; Hepatocellular carcinoma; Rupture; Insulin growth factor 2
Introduction
The development of severe hypoglycemia (defined as a circulating glucose concentration of <40 mg/dL) in critically ill patients is common [1], and may be caused, for example, by gram negative bacterial shock [2] or cardiogenic shock [3]. In addition, hypoglycemia is a paraneoplastic feature of Hepatocellular Carcinoma (HCC), along with erythrocytosis, hypercalcemia, diarrhea, and dermatitis [4,5]. Two types of HCC-associated hypoglycemia are defined: type A, mild hypoglycemia, which develops at the end stage of the tumor pathology; and type B, severe hypoglycemia, caused by large amounts of Insulin-Like Growth Factor 2 (IGF2) being released by HCC cells, a phenomenon that is termed Non-Islet Cell Tumor Hypoglycemia (NICTH) [6,7]. To date, several cases of HCC-associated NICTH have been reported [6-13]. The spontaneous rupture of HCCs is not infrequent and is a life-threatening condition that requires acute intervention, such as trans-arterial embolization for hemostasis and partial liver resection [14-16]. However, hypoglycemia has rarely been reported in patients who experience spontaneous rupture of an HCC [17]. Here, we report the case of an elderly patient who had extreme hypoglycemia (blood glucose <10 mg/dL) that was associated with the spontaneous rupture of an end-stage HCC.
Case Presentation
An 85-year-old non-diabetic woman was brought to the emergency room of our hospital in a state of shock and coma. She was not anemic, but her vital signs were: Japan Coma Scale 100, Glasgow Coma Scale E1V2M4, heart rate 63 /min, and respiratory rate 14 / min; her blood pressure and oxygen saturation were unmeasurable. In addition, she was found to be extremely hypoglycemic (blood glucose <10 mg/dL; in fact, repeated tests showed concentrations of only 1.0 mg/dL), as well as having hepatic and renal dysfunction (Table 1). She had been diagnosed with hepatitis B and C-negative HCC one year earlier, but no history of alcohol consumption was available. She and her family had declined any treatment and made no further visits to the clinic. According to her family, the patient had been unable to eat food for nearly a week prior to her hospitalization. Computed tomography performed in the emergency room revealed massive tumor nodules in both lobes of the liver and rupture of the HCC in the left lobe (Figure 1). After cardiopulmonary arrest, the patient’s circulation returned spontaneously, and intravenous glucose administration, including the rapid infusion of 40 mL of 50 % glucose, followed by continuous infusion (20 mL/hour) of 10 % glucose, returned her blood glucose concentration to normal within 4 hours, but this decreased thereafter, and she died 10 hours after admission (Figure 2).
Complete blood count
Total protein; g/dL (6.6-8.1)
7.2
WBC; /μL (3,300-8,600)
11,100
Albumin; g/dL (4.1-5.1)
2.5
RBC; x106/μL (3.86-4.92)
5.11
Renal function
Hb; g/dL (11.6-14.8)
15.5
BUN: mg/dL (8.0-20.0)
94.3
PLTc; ×103/μL (158-348)
94
Creatinine; mg/dL (0.46-0.79)
4.36
Glucose-related indices
Uric acid; mg/dL (2.6–5.5)
24.2
Blood glucose; mg/dL (73-109)
1.0
eGFR; (>60)
9
HbA1C; % (<6.2)
4.9
Inflammation-related indices
Liver-related indices
CRP; mg/dL (<0.14)
5.57
AST; U/L (13-30)
681
Procalcitonin; ng/mL (<0.05)
0.72
ALT; U/L (7-23)
101
Blood gas analysis
LDH; U/L (124-222)
1,256
pH; (7.35-7.45)
7.32
ChE; U/L (100-240)
145
BE; mEq/L (-3-+3)
-0.9
Gamma-GTP; U/L (9-32)
231
Glucose; mg/dL (70-110)
1.0
Total bilirubin; mg/dL (0.4-1.5)
3.62
Lactate; mg/dL (5-15)
61
Table 1: Laboratory data on admission.
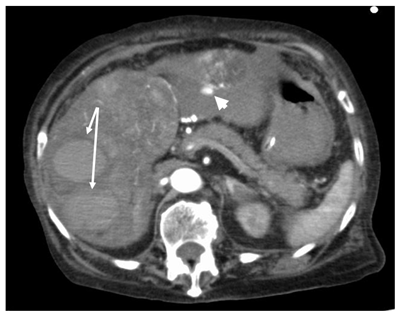
Figure 1: Contrast-enhanced computed tomography revealed widespread
Hepatocellular Carcinoma (HCC) in both lobes of the liver and a rupture of
the tumor in the left lobe. Arrows indicate intrahepatic hematomas and the
arrowhead indicates extra hepatic hemorrhage.
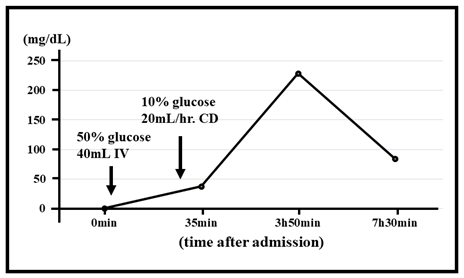
Figure 2: Clinical course of the patient’s hypoglycemia after admission.
IV: Intravenous Infusion; CD: Continuous Infusion.
At autopsy, neither insulinoma nor a gastrointestinal stromal tumor was found. Her liver weighed 1,290 g, showed cirrhosis, and was filled with moderately differentiated HCC tumor masses, one of which had ruptured (Figure 3). We also determined whether the HCC had been producing excessive amounts of IGF2 by immunohistochemical staining as previously described [9,11]. Formalin-fixed, paraffin-embedded HCC tissue was sectioned and immunostained using an anti-IGF2 antibody (anti-rabbit polyclonal antibody; Merck, Kenilworth, NJ, USA), but no expression was found (data not shown).
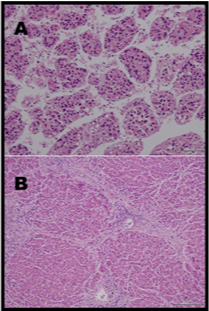
Figure 3: Histology of the patient’s liver, showing Hepatocellular carcinoma
and cirrhosis. A) Original magnification, ×200 and B) Original magnification,
×100. Immunostaining for insulin-like growth factor 2 was negative in both
samples (data not shown).
Discussion
Rupture of an HCC creates a life-threatening condition that requires acute intervention [14-16]; however, the present patient, who was in the terminal stage of the disease, could not be treated aggressively, because her family did not give their permission. The cause of the underlying hepatic cirrhosis in the patient was unknown. We considered several possible causes of her extremely severe hypoglycemia: Spontaneous rupture of the HCC [17] and a combination of type A and type B hypoglycemia [5-12]. We were unable to assay serum IGF2 or undertake western blot analysis premortem for the diagnosis of NICTH, but we immunostained liver tissue obtained at autopsy for IGF2, but the results were negative, implying that the severe hypoglycemia in this case was not HCCrelated NICTH. In addition, NICTH caused by other tumors was ruled out. Because the patient did not survive for long, detailed studies of hormone, catecholamine, and cytokine concentrations could not be performed to further investigate the cause(s) of the severe hypoglycemia.
We could not find any previous reports of HCC-related extreme hypoglycemia (blood glucose concentration <10 mg/dL) in the literature. In previous reports of HCC-associated NICTH, the blood glucose concentrations of the patients were 20–38 mg/dL [5-9,12,13]. Type A hypoglycemia is generally characterized as being mild [6,7]. For the present case, we hypothesized that the causes of the extremely severe hypoglycemia were shock induced by the rupture of the HCC; impaired glucose metabolism in the liver, associated with the loss of functional hepatocytes; consumption of glucose by the HCC; and poor nutrition, especially the lack of food intake during the week preceding hospitalization. However, we do not have specific evidence for effects of shock-related factors on the patient’s glucose metabolism. After admission, her hypoglycemia was rapidly improved by therapy, but the outcome was poor. Finally, the lack of significant anemia, despite the rupture of the HCC, might be explained by HCC-related erythrocytosis [4].
Conclusion
Spontaneous rupture of an HCC may induce severe hypoglycemia through a mechanism not involving NICTH.
Acknowledgements
The authors thank Professor Kyuichi Kadota, Shimane University Faculty of Medicine, for the histochemical staining of the HCC tissue for IGF2 and helpful comments.
Ethical Approval
This study was performed in accordance with the Declaration of Helsinki.
References
- Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: Risk factors and outcomes. Crit Care Med. 2007; 35: 2262-2267.
- Berk JL, Hagen JF, Beyer WH, Gerber MJ. Hypoglycemia of shock. Ann Surg.1970; 171: 400-408.
- Crook MA. Hypoglycemia, hypotriglyceridemia and starvation associated with cardiogenic shock. Nutrition. 2014; 30: 1093-1094.
- Luo J-C, Hwang S-J, Wu J-C, et al. Clinical characteristics and prognosis of Hepatocellular carcinoma patients with paraneoplastic syndromes. Hepatogastroenterology. 2002; 49: 1315-1319.
- Regino CA, López-Montoya V, López-Urbano F, et al. Paraneoplastic Hypoglycemia in Hepatocarcinoma: Case Report and Literature Review. Cureus. 2020; 12: e12013.
- Tsai C-Y, Chou S-C, Hsien-Ta Liu H-T, et al. Persistent hypoglycemia as an early, atypical presentation of Hepatocellular carcinoma: A case report and systematic review of the literature. Oncol Lett. 2014; 8: 1810-1814.
- Forde JJ, Ewelukwa O, Brar T, Cabrera R. Intractable Fasting Hypoglycemia as a Manifestation of Hepatocellular Carcinoma. Case Reports Hepatol. 2017; 2017: 7465025.
- Yonei Y, Tanaka M, Ozawa Y, et al. Primary Hepatocellular carcinoma with severe hypoglycemia: Involvement of insulin-like growth factors. Liver. 1992; 12: 90-93.
- Ishida S, Noda M, Kuzuya N, et al. Big insulin-like growth factor II-producing Hepatocellular carcinoma associated with hypoglycemia. Intern Med. 1995; 34: 1201-1206.
- Zhou S, Jiang L, Sun M. Recurrent hypoglycemic coma as the initial and single clinical manifestation of advanced Hepatocellular carcinoma. J Gastrointest Cancer. 2015; 46: 64-67.
- Naruse H, Shimoyama N, Satoh T, et al. [Non-islet cell tumor hypoglycemia caused by a big insulin-like growth factor II- producing hepatocellular carcinoma: An autopsy case report]. Nihon Shokakibyo Gakkai Zasshi. 2016; 113: 2057-2066.
- Rana P, Kim B. A Unique Case of IGF-2 Induced Hypoglycemia Associated with Hepatocellular Carcinoma. Case Rep Endocrinol. 2019; 2019: 4601484.
- Yu B, Douli R, SuarezJA, et al. Non-islet cell tumor hypoglycemia as an initial presentation of hepatocellular carcinoma coupled with end-stage liver cirrhosis: A case report and review of literature. World J Hepatol. 2020; 12: 519-524.
- Moris D, Chakedis J, Sun SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: A systematic review. J Surg Oncol. 2018; 117: 341-353.
- Chua DW, Koh Y-X, AllenJC, et al. Impact of spontaneous rupture on the survival outcomes after liver resection for hepatocellular carcinoma: A propensity matched analysis comparing ruptured versus non-ruptured tumors. Eur J Surg Oncol. 2019; 45: 1652-1659.
- Lai ECH, Lau WY. Spontaneous rupture of Hepatocellular carcinoma: A systematic review. Arch Surg. 2006; 141: 191-198.
- Jayet PY, Frochaux V, Qanadli S, Lamy O. [Spontaneous hemoperitoneum: Diagnosis, prognosis and possible therapeutics]. Praxis (Bern 1994). 2006; 95: 1091-1094.