Case Report
J Dent App. 2014;1(2): 23-24.
A Rare Case of Malignant Hemangiopericytoma in the Mandible
Yildirim B and Shuibat A*
Gazi University, Faculty of Dentistry Department of Oral Pathology, Ankara, Turkey
*Corresponding author: Shuibat A, Department of Oral Pathology, Gazi University, Emek 65100, Turkey
Received: May 09, 2014; Accepted: July 14, 2014; Published: July 16, 2014
Abstract
Hemangiopericytoma (HPC) is a rare malignant vascular tumor arising from mesenchymal cells with pericytes differentiation. HPC represents only 1% of the adult vascular neoplasms. This tumor usually occurs in the limbs and pelvis with only 5% in the oral cavity and pharynx. The histological appearance does not reliably predict the biologic behaviour of the tumor. Due to chances of late recurrence and metastasis, several studies recommended long-term follow-up. A case of malignant hemangiopericytoma in the mandible in a 24 years old female with 7 years follow up have been reported in this article.
Introduction
The hemangiopericytoma is a very rare tumor in the head and neck region. The first description of this tumor goes back to Scout and Murray in 1942. The tumor takes its origin from the per capillary pericytic cells of Zimmermann. These cells are present external to the endothelial cells of capillaries and venules. They may be considered as contractile cells which wrap themselves around the capillaries and venules and serve to change the caliber of their lumens. It shows variable degree of malignant potential and recurrence [1].
HPC is primarly a tumor of adult life, it is rare in infants and children. HPCs represent only 1% of all adult vascular neoplasms and 3-5% of all soft tissue sarcomas. About 15% of all HPC occur in the head and neck; in this area the sinonasal tract is the most common site followed by Orbita and nasopharynx [2,3].
HPCs may be benign (low-grade), malignant (high grade), or may occur in an intermediate form characterized by unpredictable biologic behavior [3,4]. Distinction between low and high-grade lesions is challenging on the basis of histological parameters. Tumors with benign histologic appearance have been reported to metastase [5]. An unusual case of a malignant HPC in the right mandible have been reported.
Case Report
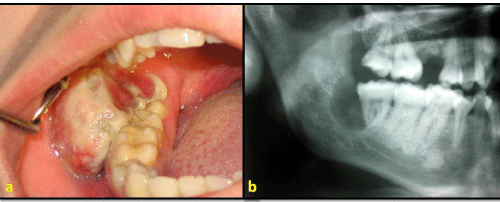
A 24 year-old female patient was referred to Gazi University Faculty of Dentistry Department of Oral and Maxillofacial Surgery with a chief complaint of pain and swelling in the right mandibular third molar area of 3 months duration. The medical history of the patient was unremarkable but the oral hygiene was poor. On clinical examination red, firm, pedunculated, non-tender, non-fluctuant swelling was present in the right mandibular third molar area, measuring approximately 5 cm in diameter (Figure 1a). The right mandibular third molar tooth was partially erupted.
Figure 1a: Red, ulcerated, pedunculated and non fluctuant lesion in the third molar area. Figure 1b: Panoramic radiograph showed partially erupted right mandibular third molar tooth associated with well-defined, round to oval radiolucent lesion.
Panoramic radiograph showed partially erupted right mandibular third molar tooth associated with well-defined, round to oval radiolucent lesion approximately 2.5 x 2,0 cm in size. Loss of cortication around the inferior alveolar canal in the effected area was observed (Figure 1b). Neither paresthesia nor palpable regional lymph node enlargement were noted. On the basis of history and clinico-radiological examination a provisional diagnosis of malignant neoplasm was given with differential diagnosis of ossifying fibroma, synovial sarcoma, fibrosarcoma, burkitts lymphoma, ewing's sarcoma, chondrosarcoma and hemangioendothelioma.
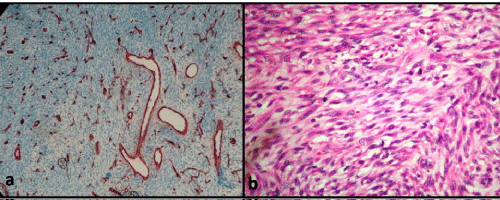
Incisional biopsy was carried out under local anesthesia and sent to oral pathology department for histopathological evaluation. The tumor was composed of haphazard tightly packed round to fusiform cells with indistinct cytoplasmic borders surrounding thin-walled blood vessels (Figure 2a). The vascular spaces ranged in size from small capillaries to large sinusoidal spaces with focal perivascular hyalinization. A staghorn vascular configuration was prevalent. 10 mitoses per 10 high-power fields (HPF) had been observed with focal evidence of cytological atipy (Figure 2b). Reticulin stain showed dense pericellular reticulin fibres surrounding small groups of cells and blood vessels and a focal positive reaction for CD 68 (a macrophage marker), while immunoreactvity was negative for EMA (epithelial membran antibody), S100 protein, α-SMA, desmin, myoglobulin. The endothelial cells of the capillaries were positive to QBEnd10 antibody (a type of CD-34 antibody, selectively associated with human progenitor cells and is expressed by vascular endothelial cells). A large hemorrahage area was observed in the intraosseous part of the tumor. There was no evidence of necrosis or giant cells. The histopathological features and immunohistochemical profile of the case was consistent with malignant hemangiopericytoma. Complete resection of the tumor was undertaken under general anesthesia with reconstruction with titanium plate and screws.In seven years of follow up, whole body bone scintigraphy, thoracic comp.
Figure 2a: Gaping vascular channels lined by single layer of endothelium and surrounded by haphazardly arranged tumor cells (SMA ABC X 200). Figure 2b: Mitoses and focal evidence of cytologic atipy ( H&E, X 200).
Discussion
Hemangiopericytoma is an uncommon tumor, which shows variation in biological behaviour that poses diagnostic and therapeutic difficulties. In general, the key to diagnosis of HPC is still the microscopic architectural pattern. Although the tumor often appears encapsulated, subsequent histological examination often demonstrated infiltration of the capsule wall with tumor cells. HPC usually arranges in a multilobular fashion. The lobules are made up of tightly packed, small, ovoid to spindle cells with indistinct cytoplasmic borders that are arranged around an elaborate vasculature. There are tightly packed cells growing around the ramifying, thin-walled, endothelium-lined vascular channels ranging from longer sinusoidal spaces to small capillaries. Classically, the vascular pattern is described as 'stag horn' or 'antler-like' in configuration [2-4]. Commonly the vessels, particularly large ones are invested with a thick coat collagen that extends into the interstitium. Reticulin staining shows lesionel vessels lined by a single layer of endothelial cells, with the pericytes lying outside the basal lamina, although they are often individually surrounded by reticulin and collagen fibers. Enzinger and Weiss reported that in some HPC, there are also focal spindle cells areas, but the spindle cells are never arranged in long bundles or fascicles as in the solitary fibrous tumor, fibrosarcoma or synovial sarcoma. In the present case, long and intersecting chords and bundles formed by spindle cells were observed. As in this case focal or diffuse fibrosis and myxoid change is a common feature in the HPC [4,5].
The radiographic feature is that of a malignant bone lesion but is not specific. Although the tumor often appears encapsulated, subsequent histological examination often demonstrated infiltration of the capsule wall with tumor cells [4,5]. HPC is made up of tightly packed, small spindle cells with indistinct cytoplasmic borders. Classically, the vascular pattern is described as 'stag horn' or 'antler-like' in configuration. Commonly the vessels, particularly large ones are invested with a thick coat of collagen with focal or diffuse fibrosis and myxoid change [5]. The blood vessels are lined with normal single layer of endothelial cells in contrast to malignant angiosarcoma, in which the vascular spaces are lined with malignant tumor cells [5,6].
Immunohistochemistry has a controversial role in the diagnosis of HPC. It is more useful in excluding other diseases as there is no specific marker for this tumor. The immunohistochemical analysis recommended by Enzinger and Stout includes vimentin, desmin, actin, CD34, CD31 and Factor VIII. Vimentin is the only marker that is consistently expressed in HPC as a constant intermediate filament [5]. The results of immunohistochemical staining of HPCs in the literature [6,7].
Hemangiopericytic pattern is not unique to the HPC. Other vascular, histiocytic, neural and smooth muscle neoplasms have to be excluded before the diagnosis of HPC. Differential diagnosis of this tumor include Solitary fibrous tumor, monophasic synovial sarcoma, infantile myofibromatosis, myopericytoma, mesenchymal chondrosarcoma, thymoma, fibrohistiocytoma, malignant peripheral nerve sheat tumor and epitheloid leimyosarcoma [7,8].
The histologic features and diagnosis of hemangiopericytomas do not always correlate with the clinical behaviour of the lesion. Tumors with benign histologic features have been reported to metastases. It has been suggested that all hemngiopericytomas have a malignant potential [9]. Enzinger and Smith proposed some criteria for malignancy which include : macroscopically larger than 5 cm, an increased mitotic rate (≥ 4 mitoses/10 HPF), a high degree of cellularity, precence of pleomorphic tumor cells and necrosis [9]. Middleton et al. demonstrated that tumors with a trabecular pattern, necrosis, mitoses, vascular invasion, and cellular atypia have higher capacity of recurrences and metastases [8,9].
Conclusion
Despite the rarity of HPC, it should be considered as one of the differential diagnosis of tumors of the head and neck. It continues to be a very diagnostic and therapeutic challenge and every diagnosed case of hemangiopericytoma could be a contriputing factor.
Financial/Nonfinancial Disclosures
The authors have reported that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
References
- Backwinkel KD, Diddams, JA. Hemangiopericytoma. Report of a case and comprehensive review of the literature. Cancer. 1970; 25: 896-901.
- Battifora H. Hemangiopericytoma: Ultra structural study of five cases. Cancer. 1973; 31: 1418-1432.
- Vogler JJ, Andavolu R, Leban SG. Malignant hemangiopericytoma of the gingiva: Report of a case. J Oral Maxillofac Surg. 1990; 48: 990-992.
- Delsupehe KG, Jorissen M, Sciot R, De Vos R, Van Damme B, Ostyn F. Hemangiopericytoma of the head and neck: A report of four cases and a literature review. Acta Otorhinolaryngol Belg. 1992; 46: 421-427.
- Weiss SW, Goldblum JR. Enzinger and Weiss'sSoft Tissue Tumors, 4th edition, St Louis, MO: Mosby, 2001. p 1004-1021.
- Florence SM, Willard CC, Palian CW. Hemangiopericytoma of the buccal region. Oral Maxillofac Surg. 2001; 59: 449-453.
- Guillou L, Fletcher JA, Fletcher CDM, Mandahl N. In: Fletcher CDM, Unni K, Mertens F editors. World Health Organization Classification of Tumors-Pathology and Genetics of Tumors of Soft Tissue and Bone. IARC Press Lyon. 2002. p. 86-90.
- Moriya S, Tei K, Notani K, Shindoh M. Malignant hemangiopericytoma of the head and neck: A report of 3 cases. J Oral Maxillofac Surg. 2001; 59: 340-345.
- Morita N, Yabuta T, Todo K, Taenaka Y. A metastatic haemangiopericytoma of the floor of the mouth. Int J Oral Maxillofac Surg. 2006; 35: 563-565.