
Case Report
J Dent App. 2015;2(2): 153-157.
Stability of the Adhesive Interface after Different Artificial Aging Techniques
Souza LC1, Apolonio FM1, Souza LP2, Carrilho MRO3 and Saboia VPA4*
1Post Graduate Program of Dentistry - Faculty of Pharmacy, Dentistry and Nursing, Federal University of Ceará, Fortaleza, Ceará, Brazil
2Department of Biology, São Paulo State University “Julio Mesquita” (UNESP), Rio Claro, São Paulo, Brazil
3Department of Oral Biology, School of Dentistry, Schulich School of Medicine & Dentistry, Western University, London, Ontario, Canada
4Department of Operative Dentistry, Federal University of Ceará, Fortaleza, Ceará, Brazil
*Corresponding author: Saboia Vpa, Department of Operative Dentistry, Federal University of Ceará, Fortaleza, Ceará, Brazil, Rua Gilberto Studart, 770 AP. 901, Bairro: CocÓ - CEP: 60.190-750
Received: September 20, 2014; Accepted: January 18, 2015; Published: January 20, 2015
Abstract
This study evaluated the stability of resin-bonded dentin interfaces using a two-step etch-and-rinse adhesive submitted to different artificial aging methods. Thirty human molars had the occlusal middle-dentin exposed and treated with Adper Single Bond 2 system, as recommended by manufacturer. Resin composite buildups were incrementally placed on bonded dentin. Specimens for micro tensile test were obtained according to the “non-trimming” technique and tested in tension, at 0.5 mm/minute, after one of the storage conditions: G1) water for 24 h (control group), G2) 10% NaOCl for 1 h, G3) continuous thermocycling (60,000 cycles at 5°-55°C for 3 months), G4) water for three months, G5) intermittent thermocycling (10,000 cycles/month at 5°- 55°C for 6 months) and G6) water for six months. Results showed that all tested methods induced a significant decrease in bond strength to dentin when compared with that observed for the control group (p<0.05). The highest decrease in bond strength values were observed for groups G2 and G3. Storage in 10% NaOCl and continuous thermocycling were the most aggressive methods to reduce the micro tensile bond strength to bonded dentin.
Keywords: Adhesives; Dentin; Sodium hypochlorite
Introduction
Perfect dentinal sealing is an elementary goal to be achieved in adhesive restorations [1]. In daily situations, restorative materials and dental restorations are commonly challenged by chemical (i.e. water, acids and enzymes) and physical/mechanic agents (i.e. changes in temperature and pH, chewing loads), which in conjunction end up accelerating the degradation of restorative materials and contributing with the disruption of dental restorations at short-term [2,3].
While not definitive, laboratorial research is fundamental to predict the complex clinical conditions that take part in the oral environment and compromise the durability of adhesive dental restorations. In this sense, in vitro aging of adhesive restorations may help one to understand the mechanisms involved in their degradation and also to find clinical and technical solutions to postpone this occurrence [4].
Storage in water has been the in vitro technique most commonly used to age adhesive restorations since the presence of water makes part, directly or indirectly, of all degrading reactions in the oral cavity [5,6]. In most of studies, the period of sample immersion may vary from few months [7-9] to some years [2,10] at the temperature of 37°C.
The aging by samples thermo cycling is commonly used to simulate the thermal changes that occur in the oral environment caused by consumption of food and drinks and even by breathing [11]. The specimens are submitted to a thermal variation that is normally driven by immersion into water baths at different temperatures [3].
Another method has been suggested to assess durability of resinbonded specimens and it consists in exposing the adhesive interface to an aqueous sodium hypochlorite solution [4]. According to Saboia et al. [12] this is a rapid and effective method of aging, which apart from challenging resinous components of resin-dentin bonds, it can also promote the degradation of dentin matrix components that have not been eventually well-impregnated by the adhesive system after dentin demineralization, a condition that seems to be more clinically realistic.
Despite promising, this chemical aging of bonds has not been sufficiently evaluated and compared with other in vitro aging methods. Thus, the present study was performed to evaluate the stability of resin-dentin bonds created by a two-step etch-and-rinse adhesive system to human dentin after different artificial aging techniques. It was hypothesized that different methods to promote in vitro aging do not differ in their ability to the bond strength of resin-bonded dentin specimens.
Methodology
Thirty freshly extracted human non-carious third molars were used in this study after obtaining the patients informed consent for their use, under a protocol approved by Federal University of Ceará (Brazil), n° 142/09. The teeth were stored in 0.01% thymol solution at 4°C for no more than 1 month. The occlusal surfaces were ground flat using 80- and 400-grit silicon carbide (SiC) to expose the deep dentin and 600-grift SiC to standardize the smear layer. Specimens were etched with 37% phosphoric acid gel for 15 s (Condac/FGM, Joinvile, Brazil), rinsed with water and dried with absorbent paper.
Single Bond 2 (3M-ESPE, St. Paul, USA) was applied in accordance with the manufacturer’s instructions (Table 1), and each bonded specimen was light-cured for 10 s using a light-curing unit (Gnatus Optilight LD Max, Ribeirão Preto, Brazil) delivering at 460mW/cm [2]. Increments of 6 mm-thick resin composite build ups (Z100, 3M-ESPE, St. Paul, USA) were placed on dentin-hybridized surfaces in increments of 2 mm that were individually light cured for 40s.
Adhesive
Composition*
Mode of application
Adper Single bond 2
(3M-ESPE, St., USA )
HEMA, bis-GMA, DMA’s methacrylate functional copolymer of polyacrylic and polyitaconic acids, water, ethanol, nanofiller, photo-initiator
- Etching: Apply phosphoric acid to enamel and dentin. Wait 15 seconds. Rinse for 15 s. Dry with absorbent paper
- Apply two consecutive coats of adhesive for 15 s with gently agitation. Gently air thin for 5 s to evaporate the solvent.
- Adhesive curing: Light cure for 10 s.
Abbreviations: HEMA – 2-hydroxyethylmethacrylate; Bis-GMA: bisphenol A diglycidyl ether dimethecrylate; DMA – dimethacrylate
*Information as received from manufacturer
Table 1: Composition of SINGLE BOND 2 and mode of application.
Teeth were randomly divided into six groups (n=5). Each tooth was longitudinally sectioned in both “x” and “y” directions, across the bonded interface, using a diamond blade in an Isomet 1000 cutting-machine (Isomet 1000, Buehler Ltd., Lake Bluff, USA) to obtain beams with cross-sectional areas of approximately 0.9 mm2, in accordance with “non-trimming” technique (Figure 1).
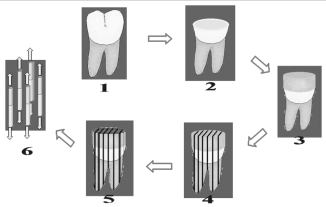
Figure 1: Schematic demonstration of the acquisition of specimens for
Microtensile Bonding test (μTBS).
Aging of specimes for microtensile test
Beams were treated according to the following groups: group 1 (G1): 24 h of storage in distilled water at 37°C (control group); group 2 (G2): 24 h of storage in water followed by exposure for 1 h at room temperature to 10% NaOCl; group 3 (G3): after 24 h of storage, bonded specimens were subjected to 60,000 of continuous thermocycles, which took approximately 3 months (approximately 20,000 cycles per month); group 4 (G4): storage in water at 37°C for the same period of time as thermocycling was performed in G3; group 5 (G5): specimens were subjected to 60,000 in intermittent thermocycles over a total period of 6 months (10,000 cycles in approximately 15 days plus storage in water for the remaining 25 days; this procedure was carried out six times); and group 6 (G6): storage in water at 37°C for 6 months. For thermocycling procedure the beans were placed in a thermocycling machine (THE-1100 Thermocycler; SD Mechatronik Gmbh, Feldkirchen-Westerham, Germany) in distilled water baths for 20.000 cycles of 5°C to 55°C with a dwelling time of 60 seconds in each bath.
Microtensile bonding test (μTMS)
Each beam was individually fixed to a custom-made testing jig with cyanoacrylate glue (Super Bonder Flex Gel; Loctite, São Paulo, Brazil). The specimen was then subjected to tensile load at a crosshead speed of 1.0 mm/min until failure (Instron 4440, Canton, USA). For specimens with premature failure was assigned a value of 0 MPa. A schematic presentation of the preparation of specimens for the μTBS test is illustrated in Figure 1.
Interfacial analysis by light microscopy
Twelve additional teeth were processed for interfacial silver nitrate evaluation. Specimens were prepared as previously described, sectioned in 1mm-thick slices and submitted to each of the aging protocols (n=2). The slices were covered with nail varnish (Risquè, Niase, São Paulo, Brazil), leaving 1 mm2 exposed at the interface and immersed in 50 wt% ammoniacal silver nitrate (AgNO3) solution (pH 9.5). After immersion in the trace solution, specimens were then thoroughly rinsed in distilled water and were immersed in photo developing solution (Kodak, Carestream Dental, New York, USA).
The silver nitrate-impregnated specimens were fixed on glass slides using cyanoacrylate glue and flattened with SiC papers on increasing fine grits (600, 800, 1200 and 2400) in a polisher under running water (Aropol 2V – Arotec, São Paulo, Brazil). Images of all interfaces at 400x magnification under light microscopy (Nikon Eclipse E 800, Tokyo, Japan) were obtained and silver nitrate representative images were chosen for each tested group.
Statistical Analysis
One-way ANOVA and the Tukey-Kramer test were used to analyze the μTBS data statistically at a significance level of a = 0.05. Silver nitrate evaluation was only performed qualitatively. The program Graph Pad Prism 5.0 (Graph Pad Software, San Diego, USA) has been used for all statistical analyses.
Results
Microtensile bonding test
The control group (Group 1) showed the highest bond strength values compared to other groups (p<0.0001). The storage in water for 3 (Group 4) and 6 months (Group 6) reduced significantly the bond strength compared to the control group, but no difference was found between that two aging protocols (p>0.05) (Table 2).
Aging group
Microtensile bond
Strength (MPa)
Group 1
Water storage for 24 h
35,5 ± 14,9a
[5/127]
Group 2
10% NaOCl for 1 h
12,43 ± 5,43d
[11/113]
Group 3
60,000 thermo cycles for 3 months
(Continuous thermo cycling)
14,07 ± 6,54d
[21/96]
Group 4
Water storage for 3 months
25,83 ± 8,93b
[10/101]
Group 5
60,000 thermo cycles for 6 months
(Intermittent thermo cycling)
19,30 ± 7,40c
[11/70]
Group 6
Water storage for 6 months
25,39 ± 10,25b
[7/105]
[Number of premature failed sticks/number of intact sticks tested]. Groups identified by different superscript letters are significantly different (P < 0.05).
Table 2: Mean ± standard deviation of micro tensile bond strength test results of adhesive interfaces bonded with Single Bond 2 after different in vitro aging conditions.
Thermocycling significantly reduced the bond strength compared to the control group and to both groups stored only in water. Moreover the continuous thermocycling aging protocol (Group 3) rendered a mean bond strength statistically lower when compared with that provided after an intermittent thermo cycling (Group 5) (p<0.05) (Table 2).
Storage in 10% NaOCl (Group 2) and the continuous thermocycling (Group 3) were similar (p>0.05) and reduced by almost 65% and 61% the bond strength of the interface resin/dentin compared to the control group, respectively. They were the most challenging aging method to degrade resin-dentin bonded interfaces (Table 2).
Interfacial analysis by light microscopy
Observation of adhesive interfaces produced with the etch-andrinse system Single Bond after twenty-four hours of the bonding procedures (control group) exhibited minimal silver uptake (Figure 2A). Aging in NaOCl for 1h resulted in bonded interfaces extensively and homogeneously impregnated with reduced silver deposits (Figure 2B). Thermo cycled specimens, under both tested conditions (i.e. continuous and intermittent), showed a significant increase in silver deposits along the adhesive interface (Figure 2C and 2E). After 3 and 6 months of storage in water, the adhesive interface showed larger deposits of silver grains (compared with the control) that were mainly localized at the bottom of the hybrid layer (Figure 2D and 2F, respectively).
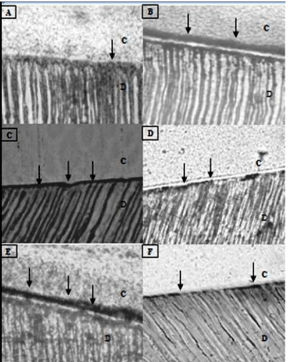
Figure 2: Light micrographs showing representative views of nano leakage
interfacial expression. C, composite resin; D, dentine; Pointers, silver
deposits. (A) Adhesive interface produced by SINGLE BOND after 24 h in
water (control group) showing minimal silver uptake. (B) Adhesive interface of
the specimen aged in sodium hypochlorite (NaOCl) for 1 h showing extensive
and homogeneous silver deposits throughout the adhesive interface (C e
E) Adhesive interfaces of thermo cycled specimens (group 3 e 5) showing
significant increases in silver deposits along the adhesive interface. (D e F)
After 3 and 6 nths of immersion in water, the adhesive interface showed an
increase in size of these silver deposits at the bottom of the hybrid layer,
although their amounts were small.
Discussion
This study showed that all specimens submitted to aging methods showed a significant reduction in bond strength compared to the control group. The effect of aging can also be seen with a massive impregnation of silver, meaning higher number of micro porosities, within aged hybrid layers in comparison with control, non-aged hybrid layers (Figures 1-6). Nevertheless, degradation of these in vitro hybrid layers either by reduction of their intrinsic strength or increase in porosities varied remarkably according to the aging protocol, being the immersion in 10% NaOCl and the continuous thermocycling the most degrading protocols. These results, therefore, lead us to reject the anticipated hypothesis that different methods to promote in vitro aging do not differ in their ability to the bond strength of resinbonded dentin specimens.
It is well known that the degree of envelopment of collagen fibrils is different depending on the type of bonding agents [6]. For total-etch adhesives, i.e. Single Bond used in this study, a decreasing gradient of resin monomer diffusion within the acid-etched dentin [13] results in incompletely infiltrated zones along the bottom of the HL that contain denuded collagen fibrils [1,14,15] in the demineralized zone of dentin created by the discrepancy between the depth of acid etching and resin infiltration. Morphologically, the exposed demineralized dentine zone at the base of the hybrid layer resulting from incomplete resin impregnation within the collagen network may be the major site of bond susceptibility to hydrolytic degradation [16,17].
It has been speculated that NaOCl solution is responsible for accelerating the deterioration of the organic matrix of resin-dentin bonded interfaces that were not perfectly impregnated with the adhesive resin during hybridization of acid-etched dentin [18]. NaOCl solution acts by forming superoxide radicals in the aqueous solution, which induces oxidation phenomena and fragment the collagen fibrils unprotected by resin monomers, thus affecting bond integrity [19]. The protocol of 1 hour storage 10% NaOCl used in this work was chosen based on two points: (1) there is no difference among 1 and 3 h of storage [12] suggesting an accelerated rate of collagen destruction at the first hour and (2) the storage for 5 hours could promote resin dissolution at the hybrid layer, and its effect depends on the adhesive system used [20]. The deleterious effect of NaOCl shown by the reduction of bond strength seen in this study is in agreement with others [12,17,18,21].
Thermocycling (group 3 and 5) reduced significantly the bond strength of the interface resin/dentin, however continuous thermocycling (group 3) showed a reduction greater than the intermittent thermocycling (group 5) and was similar to the storage in 10% NaOCl 1 h (group 2). In artificial aging, the effect induced by thermo cycling, can occur by two mechanisms: (1) hot water can speed up the hydrolysis and decomposition of interface components, and (2) a repetitive thermal contraction and expansion stress can be generated [22]. These stresses generated at the tooth–resin interface during thermo cycling [3,17] synergistically enhance the hydrolytic degradation of the adhesive and collagen fibrils at the base of the hybrid layer [1], thereby weakening the physical properties of the resin–dentine bond [23]. We speculate that in the continuous thermocycling, the changes in temperature without interruption may have caused more stress on the resin/dentin interface, leading to a greater reduce on bond strength.
Thermo cycling is an aging approach commonly used in tracer penetration protocols, shear and tensile bond strength tests either for dental materials or resin-bonded interfaces testing [11], however there is a lack of standardization in the parameters used for performing it, which makes the interpretation of results quite subjective and comparison between studies imprecise [4]. The ISO TR 11450 standard [24] (1994) indicates that a thermocycling regimen comprising 500 cycles in water between 5° and 55° C would be appropriate to age the specimens artificially. It was considered that 10,000 cycles corresponds approximately to 1 year of in vivo functioning [11], rendering 500 cycles, as proposed by the ISO standard, very minimal to mimic long-term bonding effectiveness [2]. Other studies also have shown that a large number of thermocycling (20,000 to 50,000) has influence on the degradation on the resin/dentin interface [23,25].
In order to investigate the role of each individual aging phenomenon, thermal changes and hydrolytic degradation, we compared thermo cycled specimens (groups 3 and 5) with those stored in water at 37°C for the same time-period (groups 4 and 6). Hydrolysis is a chemical process that breaks covalent bonds between the polymers by addition of water to ester bonds, resulting in loss of the resin mass: this is considered as one of the main reason for resin degradation within the hybrid layer, contributing to the reduction in bond strengths created by dentin adhesives over time [9]. Moreover, water sorption caused a significant decrease in the modulus of elasticity of the resins that is thought to contribute to reductions in bond strength, independent of resin hydrolysis [26].
The highest bond strength reduction in groups thermo cycled (groups 3 and 5) compared to those stored in water at 37°°C for the same time-period (groups 4 and 6) could be attributed both to the effects of thermal changes on the adhesive interface and to the hydrolytic degradation phenomena [21].
Conclusion
All methods of aging affected the stability of the adhesive interface resulting in reduction in bond strength. Storage in 10% NaOCl and continuous thermocycling are the most aggressive methods to reduce the micro tensile bond strength to bonded dentin.
References
- Hashimoto M, Ohno H, Sano H, Tay FR, Kaga M, et al. Micro morphological changes in resin-dentin bonds after 1 year of water storage. J Biomed Mater Res 2002; 63:306-311.
- De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, et al. Four-year Water Degradation of Total-etch Adhesives Bonded to Dentin. J Dent Res 2003; 82:136-140.
- De Munck J, Van Landuyt K, Coutinho E, Poitevin A, Peumans M, et al. Micro-tensile bond strength of adhesives bonded to Class-I cavity-bottom dentin after thermo-cycling. Dent Mater 2005; 21: 999-1007.
- Amaral FL, Colucci V, Palma-Dibb RG, Corona SA. Assessment of in vitro methods used to promote adhesive interface degradation: a critical review. J Esthet Restor Dent 2007; 19: 340-353.
- Örtengren U, Anderson F, Elgh U, Terseliusb B, Karlssona S. Influence of pH and storage time on the sorption and solubility behavior of three composite resin material. J Dent 2001; 29: 35-41.
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, et al. Dental adhesion review: aging and stability of the bonded interface. Dent Mater 2008; 24: 90-101.
- Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Effects of storage media on mechanical properties of adhesive systems. Am J Dent 2004; 17: 104-108.
- Okuda M, Pereira PN, Nakajima M, Tagami J. Relationship between nano-leakage and long-term durability of dentin bonds. Oper Dent 2001; 26: 482-490.
- Armstrong SR, Keller JC, Boyer DB.The influence of water storage and C-factor on the dentin-resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent Mater 2001; 17: 268-276.
- Fukushima T, Inoue Y, Miyazaki K, Itoh T. Effects of primers containg N-methylolacrylamide or N-methylolacrylamide on dentin bond durability of a resin composite after 5 years. J Dent 2001; 29: 227-234.
- Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent 1999; 27: 89-99.
- Saboia VP, Nato F, Mazzoni A, Orsini G, Putignano A, et al. Adhesion of two-step and etch-and-rise adhesive on collagen-depleted dentin. J Adhes Dent 2008; 10: 419-422.
- Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res 2002; 59: 46-55.
- Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 2003; 24: 3795-3803.
- Spencer P, Wang Y, Katz JL. Identification of collagen encapsulation at the dentin/adhesive interface. J Adhes Dent 2004; 6: 91-95.
- Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, et al. In vivo degradation of resin–dentin bonds in humans over 1 to 3 years. J Dent Res 2000; 79: 1385-1391.
- Hashimoto M, Tay FR, Ohno H, Sano H, Kaga M, et al. SEM and TEM analysis of water degradation of human dentinal collagen. J Biomed Mater Res 2003; 66: 287–298.
- De Munck J, Ermis RB, Koshiro K, Inoue S, Ikeda T, et al. NaOCl degradation of a HEMA-free all-in-one adhesive bonded to enamel and dentin following two air-blowing techniques. J Dent 2007; 35: 74-83.
- Yamauti M, Hashimoto M, Sano H, Ohno H, Carvalho RM, et al. Degradation of resin-dentin bonds using NaOCl storage. Dent Mater 2003; 19: 399-405.
- Toledano M, Osorio R, Osorio E, Aguilera FS, Yamauti M, et al. Durability of resin-dentin bonds: effect of direct/indirect exposure and storage media. Dent Mater 2007; 23: 885-892.
- Saboia VP, Silva FC, Nato F, Mazzoni A, Cadenaro M, et al. Analysis of differential artificial ageing of the adhesive interface produced by a two-step etch-and-rinse adhesive. Eur J Oral Sci 2009; 117: 618-624.
- Sampaio PC, de Almeida Júnior AA, Francisconi LF, Casas-Apayco LC, Pereira JC, et al. Effect of conventional and resin-modified glass-Ionomer liner on dentin adhesive interface of class I cavity walls after thermocycling. Oper Dent 2011; 36: 403-412.
- Aguilar LT, Rezende NP, Reis A, Loguercio AD, Grande RH, et al. Tensile bond strength of adhesive systems effects of primer and thermocycling. Braz Oral Res 2002; 16: 37-42.
- ISO. Guidance on testing of adhesion to tooth structure. International Organization for Standardization Geneva (Switzerland) 1994; TR 11405, 1-14.
- Miyazaki M, Sato M, Onose H, Moore BK. Influence of thermal cycling on dentin bond strength of two step bonding systems. Am J Dent 1998; 11: 118-122.
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, et al. Effects of resin hydrophilicity on water sorption and hanges in modulus of elasticity. Biomaterials 2005; 26: 6449-6459.