
Research Article
J Dent App. 2015; 2(7): 269-273.
Experimental Study on Human Demineralized Dentin Matrix as rhBMP-2 Carrier In Vivo
In-Woong Um¹*, Woo-Jin Cho² and Young-Kyun Kim³
¹Director, R&D Institute, Korea Tooth Bank, Seoul, Korea
²Researcher, R&D Institute, Korea Tooth Bank, Seoul, Korea
³Professor, Department of Oral and Maxillofacial Surgery, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea
*Corresponding author: In-Woong Um, DDS, PhD, CTBS, R&D Institute Korea Tooth Bank, Seoul-In Dental Clinic, Korea
Received: June 16, 2015; Accepted: September 10, 2015; Published: September 12, 2015
Abstract
Purpose: This study examined the bone induction capacity of human demineralized dentin matrix as an rhBMP-2 carrier.
Method: Total 40 nude mice weighing 15-20g were used for the study. Two types of scaffold were selected as a carrier of rhBMP-2: Inorganic bovine bones, tricalcium phosphate (TCP) as the control group where Autogenous tooth bone grafts material (AutoBT), a human demineralized dentin matrix (DDM), as the experimental group. Laboratory animals were sacrificed at two and four weeks after the implanting two rhBMP-2 carriers into intramuscular pouch. The evaluation of the specimen was divided into four compartments: field phenomenon, cell number (osteoblast, firbroblast), osteoid formation, and maturation, and compared two carriers histologically.
Result: Field phenomenon: Both groups have excellent fibrous encapsulations around particles; Cell number (osteoblast, fibroblast): The early cellular reaction on the surface of particles was superior in the DDM group; Osteoid formation: more osteoid was deposited on the DDM group; Maturation: The DDM group showed compact osteoid bridges and dense fibrous connective tissue filled between each particle, but the TCP group showed that the ostoid deposited seemed to be transformed into fatty tissues after 4 weeks.
Conclusion: Both the DDM/rhBMP-2 and TCP/rhBMP-2 induced bone in intramuscular pouch of nude mice, but more bone was formed in DDM/rhBMP-2 group.
Keywords: Bone induction; Demineralized dentin matrix; DDM; AutoBT
Abbreviations
DDM: Demineralized Dentin Matrix; AutoBT: Autogenous Tooth Bone Graft Material; Inorganic Bovine Bones; TCP: Tricalcium Phosphate; BMP: Bone Morphogenetic Protein.
Introduction
In 1967, there was a report that rabbit demineralized matrices (DDM) induced bone formation in the intramuscular pockets [1,2]. Since the result indicated that Bone Morphogenetic Proteins (BMPs) were involved in dentin matrix, there have been several studies to examine the animal DDM could induce ectopic bone formation in subcutaneous and intramuscular pockets in rodents [3,4].
In 2005, human DDM particles finally succeeded to induce ectopic bone and cartilage [5]. And continuous researches had developed human autogenous demineralized dentin matrix (AutoBT) that has been actively used in Korea and Japan for the past 5 years [6]. Also, another report indicated that DDM induced bone and cartilage independently while demineralized bone matrix (DBM) induced cartilage, bone and marrow at 4 weeks in the back skin of nude mice [7]. Lastly, there was a report that bone and cartilage was induced in nude mice using AutoBT by itself [8].
On the other side, a report in 1998 suggested that root dentin prepared from extracted teeth could be recycled for using as a carrier of rhBMP-2 because it induces new bone formation in the periodontium while another report showed that the osteoinductive matrices of human DDM particles could be effective as a carrier of rhBMP-2 for bone engineering later in 2005 [5, 9].
Therefore, the purpose of this study is to evaluate the bone induction capacity of DDM when fixed with rhBMP-2 whether DDM could act as anrhBMP-2 carrier.
Materials and Methods
Two types of scaffold were selected as a carrier of rhBMP-2 (CowellMedi): AutoBT (human DDM, Korea Tooth Bank, Seoul, Korea) and TCP (CowellMedi, Busan, Korea). rhBMP-2-soaked TCP (TCP/rhBMP-2) was used for the control group (n=20) where rhBMP-2-soaked DDM (DDM/rhBMP-2) was the experimental group (n=20).
Preparations of DDM
An extracted human tooth was soaked in 70% ethyl alcohol and cleaned by removing foreign substances such as restoration materials, root canal and prosthetics as well as soft tissues of periodontium, pulp and caries. After dividing cleaned tooth into crown and root, the root portion was collected and prepared for partially demineralized dentin matrix (DDM) as reported previously.0.3 to 0.8 mm crushed particles were soaked in distilled water and hydrogen dioxide solution and the remaining foreign substances were removed by ultrasonic cleaner. The cleaned particles were dehydrated with ethyl alcohol and went through defatting using ethyl ether solution. The particles were then decalcified for 2 hours in 0.6 N HCl by repeated30-minute periods [8,10]. After the demineralization, the dentinal tubules and compact peritubular dentinal collagens are widened and loosened respectively (Figure 1A, 1B).
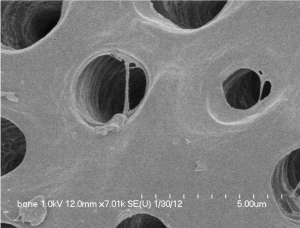
Figure 1A: Normal dentin before treatment. Courtesy from: Lee HJ.
Quantitative analysis of proliferation and differentiation of MG-63 cell line on
the bone grafting material using human tooth. PhD thesis. School of Dentistry,
Seoul National University. 2011.
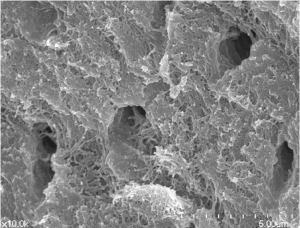
Figure 1B: Surface of DDM after demineralization. Courtesy from: Lee HJ.
Quantitative analysis of proliferation and differentiation of MG-63 cell line on
the bone grafting material using human tooth. PhD thesis. School of Dentistry,
Seoul National University. 2011.
Preparation of DDM/rhBMP-2
The 2 mg/ml rhBMP-2 (Cowell BMP, Busan, Korea) was fixed to 0.03g of DDM by placing both into 15-ml conical tubes. The mixtures were frozen in a deep freezer at –70 °C, and then fixed in a lyophilizer (ILShin Lab, Seoul, Korea).The rhBMP-2 was provided by the Research and Development Institute of Cowellmedi (Busan, Korea) [10].
Figure 2A: DDM before rhBMP-2 fixation.

Figure 2B: DDM after rhBMP-2 fixation.
Preparation of TCP/rhBMP-2
The rhBMP-2 was fixed to inorganic bovine bones, tricalcium phosphate (TCP). Inorganic bovine bones, microporous BCP (particle type, 0.5–1.0mm, 70% porosity; Bio-C, Cowellmedi) with a HA:b-TCP ratio of 3:7 was used as a rhBMP-2 carrier. Briefly, the rhBMP-2 solution (0.67mL in 1.5mg/mL buffer) was added to 1 g of TCP particles and lyophilized in a freeze dryer [11].
Figure 3A: TCP before rhBMP-2 fixation [10].

Figure 3B: TCP after rhBMP-2 fixation [10].
Surgical procedure
In this study, 40 nude mice weighing 15-20 g were used as experimental animals. These animals were bred with a free supply of cubed diet and water under average ambient temperature condition of 22 °C, and a 12-hour shade cycle. The experiment was approved by Institutional Animal Care and Use Committee.
We gave general anesthesia through intra-peritoneal administration of pentobarbital sodium (Nembutal,43 mg/kg; Dainabot Co., Tokyo, Japan) that had been diluted in sterile water for injection, then disinfected and isolated the surgical site. Incision was made in dorsum, and a pouch was formed in muscle at both sides. In the control group (n=20 mice), 20 μg grafts of TCP/rhBMP-2 (concentration=0.05 mg/ml) were applied while 20 μg grafts of DDM/rhBMP-2 (concentration=0.05 mg/ml) were applied in the experimental group (n=20 mice) to evaluate at 2 and4 weeks of implantation.
Histomorphometric analysis
Laboratory animals were sacrificed at two and four weeks after the implantation. The specimens were procured en bloc and fixed in 10% neutral buffered formalin, decalcified with 10% formic acid, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). For histomorphometric analysis, tissues of the implanted area were divided into four compartments: field phenomenon, cell number (osteoblast, fibroblast), osteoid formation, and maturation.
The number of bone forming cells was calculated by dividing total cell numbers on the dentin surface by the number of dentin particles that were observed in the specimen. Cell number, Osteoid formation, and maturation were calculated by designating TCP/rhBMP-2 group as a standard 50%.
Result
Field phenomenon (soft tissue)
Both groups have excellent fibrous encapsulations around particles; the fibrous tissue compaction between particles in DDM group was less than TCP group. For the DDM group, some voids were seen in the central area (Figure 4A). On the other hand, there were no voids between particles in the TCP group (Figure. 4B).
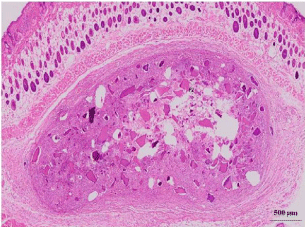
Figure 4A: DDM/rhBMP-2 after 2 weeks.
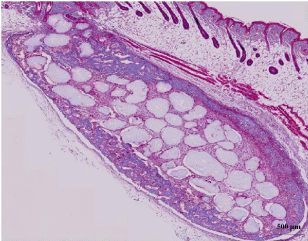
Figure 4B: TCP/rhBMP-2 after 2 weeks.
Cell number (osteoblast, fibroblast)
The early cellular reaction on the surface of particles was superior in the DDM group than in the TCP group after 2 weeks of the implantation. Fibroblasts around DDM particles seemed to be transformed into osteoblast, which produced new osteoid and was deposited on surface of DDM. The characteristic palisade arrangement of cell along with the osteoid was found (Figure. 5A). In contrary, dense fibrous connective tissue bridges between the TCP particles were seen as the evidences of biocompatibility (Figure. 5B).
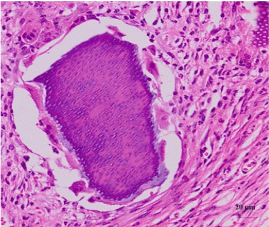
Figure 5A: DDM/rhBMP-2 after 2 weeks.
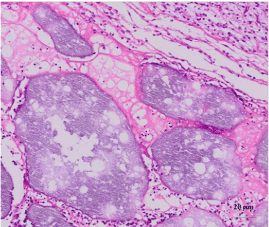
Figure 5B: TCP/rhBMP-2 after 2 weeks.
Osteoid production
After 2 weeks of the implantation, more osteoid was deposited on the DDM group than the TCP group. The texture of osteoid was different from each other in terms of the density of connective tissue. We could find heavily deposited osteoid around the DDM group while the osteoid in TCP group was thin and sparse (Figure 6A, 6B).
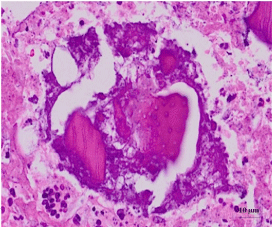
Figure 6A: DDM/rhBMP-2 after 2 weeks.
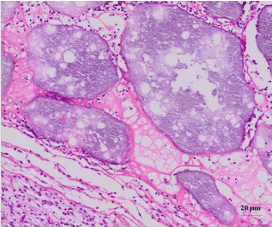
Figure 6B: TCP/rhBMP-2 after 2 weeks.
Maturation
The DDM group showed compact osteoid bridges and dense fibrous connective tissue filled between each particle, but the TCP group showed that the osteoid deposited seemed to be transformed into fatty tissues after 4 weeks (Figure 7A, 7B).
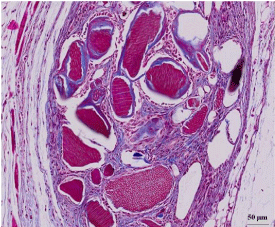
Figure 7A: DDM/rhBMP-2 after 4 weeks.
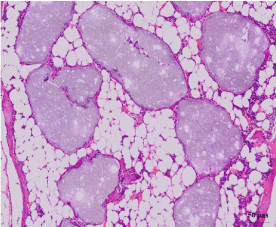
Figure 7B: TCP/rhBMP-2 after 4 weeks.
The histological evaluations of DDM/rhBMP-2 and TCP/ rhBMP-2 were summarized in (Figure 8).
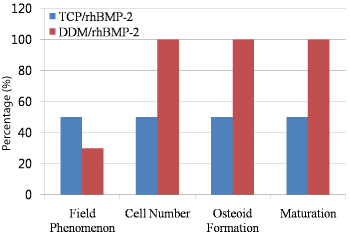
Figure 8: The summary of the histological evaluations of DDM/rhBMP-2 and
TCP/rhBMP-2.
Discussion
Even if several studies revealed the osteoinductivity of the rat and rabbit dentin, human DDM of vital teeth origin could be less osteoconductive than DDM derived from short-lived animals such as rat, rabbit, mouse, pig, and cattle [1-4, 9, 12, 13].
In1991, a protein with BMP-like activity was isolated from human dentin and evaluated, but the NH2-terminal amino acid sequence did not resemble to that of the known BMP family [14]. Thus, Dentinmatrix- derived BMP and bone-matrix-derived BMP could not be identical each other, but have the similar osteoinductive action in vivo [15].
The calcified dentin matrix(CDM)’s lack of inductive properties had been related to the inhibition of BMP release due to apatite crystal, the dense tube-structure (Figure1A), the trace amount of dentin BMPs, and the inactivation of BMPs occurred during the fabrication procedures. In other words, this could be explained by the fact that such mineral acted as a barrier to chemotactic and morphogenic molecules of dentin while acid initiated a demineralization process that exposes osteoinductive molecules like BMPs. Thus it indicated that demineralization of dentin matrix would be a key determinant factor in subsequent inductive and conductive potentials. Studies had reported that demineralization of dentin exposes the sequestrated BMPs in the insoluble collagen of the dentin matrix. Therefore, decalcified dentin has revealed better bone induction activity due to the activation of BMPs, which bind to collagen matrices through the demineralization process [16].
In 2003, Demineralized dentin particles from human adult extracted teeth succeeded to induce bone and cartilage independently in subcutaneous tissues of nude mice at 4 wks of implantation (0.4-0.8mm) [17] and then the report in 2005 confirmed that completely demineralized human dentin matrix including small patches of cementum induced bone and cartilage independently in subcutaneous tissues of nude mice[5]. Furthermore, several studies on the osteoinductivity of AutoBT, a partially demineralized dentin matrix, had been reported for the last decade [8, 18]. However, the attempt to delineate the rhBMP-2 in AutoBT via conventional method was failed even though the AutoBT showed excellent osteoinductivity in vivo experiment and human clinical studies. It could be due to the trace amount of rhBMP-2 in dentin matrix as well as the denaturation during the fabrication procedures [19].
Recent studies provided the evidence that addition of rhBMP-2 to demineralized dentin would accelerate its osteoinductivity [5,6,20]. Thus we previously studied and reported time-course release study of rhBMP-2from carriers in rats to evaluate AutoBT as rhBMP-2 carrier. And a statistically significant large amount of rhBMP-2 was released from AutoBT compared to TCP (p<0.05). And the expression of osteonectin and bone formation of AutoBT was superior to the TCP. Therefore, the result suggested that DDM could be an effective carrier of rhBMP-2 [10].
In this experiment, Fibrous encapsulation and connective tissue filling showed the initial biocompatibility of both experimental and control group. And the cellular activities shown in this experiment was consistent with that of our previous report that a larger amount of rhBMP-2 was released from AutoBT than TCP. More fibroblasts, which were transformed into osteoblast by rhBMP-2, were released from AutoBT than TCP. Consequently, more osteoid in AutoBT was deposited and involved in the maturation procedure than in that of TCP [10].
Conclusion
In conclusion, both the DDM/rhBMP-2 and TCP/rhBMP-2 induced bone in intramuscular pouch of nude mice, but more bone was formed in DDM/rhBMP-2 group. Therefore, within the limitation of this study, we could suggest that the DDM could be an effective carrier of rhBMP-2 for bone engineering. The additional study would be needed by implanting DDM/rhBMP-2 in skeletal site to support the conclusion of this experiment.
References
- Yeomans JD and Urist MR. Bone induction by decalcified dentin implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967; 12: 999-1008.
- Bang G and Urist MR. Bone induction in excavation chambers of decalcified dentin. Arch Surg. 1967; 94: 781-789.
- Huggins C, Wiseman S and Reddi AH. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med. 1970; 132: 1250-1258.
- Butler WT, Mikulski A, Urist MR, Bridges G and Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977; 56: 228-232.
- Murata M. Bone engineering using Human Demineralized Dentin Matrix and Recombinant Human BMP-2. International Symposium of Maxillofacial & Oral Regenerative Biology in Okayama. 2005; 80-81
- Kim, YK, Um IW and Murata M. Tooth bank system for bone regeneration-Safety report. J Hard Tissue Biol. 2014; 23: 371-376.
- Murata M, Akazawa T, Takahata M, Ito M, Tazaki J, Hino J, et al. Bone induction of human tooth and bone crushed by newly developed automatic mill. J Ceram Soc JPN. 2010; 118: 434-437.
- Kim KW. Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles. Maxill of acPlast Reconstr Surg. 2014; 36: 50-56.
- Ike M and Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998; 24: 124-132.
- Kim YK, Um IW, An HJ, Kim KW, Hong KS and Murata M. Effects of Demineralized Dentin Matrix Used as an rhBMP-2 Carrier for Bone Regeneration. Journal of Hard Tissue Biology. 2014; 23: 415-422.
- Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010; 31: 3512–3519.
- Kawai T and Urist M R. Bovine tooth-derived bone morphogenetic protein. J Dent Res. 1989; 68: 1069-1074.
- Inoue T, Deporter DA and Melcher AH. Induction of chondrogenesis in muscle, skin, bone marrow and periodontal ligament by demineralized dentin and bone matrix in vivo and in vitro. J Dent Res. 1986; 65: 12-22.
- Bessho MK, Tagawa T and Murata M. Analysis of bone morphogenetic protein derived from human and bovine bone matrix. Clinical Orthopaedics and related Research. 1991; 268: 226-234.
- Bessho MK. Human Dentin–matrix-derived Bone Morphogenetice Protein. J DENT RES. 1991; 70:171-175.
- Mahdi K, Majid G, Negar S, Gholam RS, Shahriar A and Reza A. Effects of Fresh Mineralized Dentin and Cementum on Socket Healing. A Preliminary Study in Dogs. J Korean Assoc Oral Maxillofac Surg. 2015; 41: 119-123.
- Murata M, Sato D, Taira T, Sasaki T and Arisue M. Bone and cartilage induction in nude mice by human demineralized dentin matrix. J hard Tissue Biol. 2003; 11: 110-114.
- Kim YK, Lee JK, Kim KW, Um IW and Murata M. Advances in Biomaterials Science and Biomedical Applications, ed. Pignatello, R., Chapter 16 “Healing Mechanism and Clinical Application of Autogenous Tooth Bone Graft Material,” (In Tech publisher, Croatia). 2013: 405-435.
- Kim YK, Lee JH, Kim KW, Um IW, Murata M and Ito K. Analysis of Organic Components and Osteoinductivity in Autogenous Tooth Bone Graft Material. J Korean Assoc Maxill of acPlast Reconstr Surg. 2013; 35: 353-359.
- Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP-2 accelerates bone induction in subcutaneous and intramuscular tissues. JCeram Soc JPN. 2010; 118: 438-441.