
Research Article
J Dent App.2016; 3(1): 297-302.
The Influence of Magnetic Resonance on the Microstructure and Shear Bond Strength of Silver Palladium Dental Alloy and Feldspathic Porcelain
Sakrana AA¹*, El-Bediwi AB², Al-Ragaei D² and Özcan M³
¹Department of Fixed Prosthodontics, Faculty of Dentistry, Mansoura University, Egypt
²Metal Physics Laboratory, Physics Department, Faculty of Science, Mansoura University, Egypt
³Dental Materials Unit, University of Zurich, Center for Dental and Oral Medicine, Clinic for Fixed and Removable Prosthodontics and Dental Materials Science, Switzerland
*Corresponding author: Amal Abdelsamad Sakrana, Department of Fixed Prosthodontics, Faculty of Dentistry, Mansoura University, Mansoura, Egypt
Received: February 17, 2016; Accepted: March 18, 2016; Published: March 21, 2016
Abstract
Chipping and delaminating of veneering ceramics of metal-ceramic restorations are the long dilemma for the patients and dentists. Therefore, this study aimed to detect the effect of Magnetic Resonance Imagining (MRI) signals on the microstructure and mechanical properties of silver-palladium dental alloys and its bond strength to ceramic veneer.
Materials and Methods: 60 self-cured acrylic resin discs (15 X 1 mm) used to prepare silver-palladium specimens. The specimens were airborne abraded with 110 μm Al2O3 then cleaned for 3 minutes and divided into two groups. The first group (N=30) was used for Vickers hardness test, X-ray diffraction analysis and Scanning Electron Microscope (SEM) for microstructure evaluation of metal specimens without MRI exposure and after 15 and 30 minutes exposure. Ceramic veneer was applied to the second group (N=30) then were subjected to 6000 thermocycles in distilled water between 5°C and 55°C. The veneered alloy specimens were randomly divided into 3 groups according to MRI exposure time; no exposure (control), 15 and 30 minutes. The specimens were subjected to continuous shear loading till failure. One way Analysis of Variance (ANOVA) was used to compare the mean of variables at different MRI exposure times.
Results: Increasing exposure time to MRI for 30 minutes significantly decreased the shear bond strength (20.74±0.31) compared to the control group (33.51±0.25). Vickers hardness of silver-palladium alloy was significantly decreased after 30 minutes MRI exposure compared to control group (334.66±0.28 and 233.13±0.36), respectively. SEM analysis showed a considerable changes after MRI exposure that proved by the X-ray diffraction analysis.
Conclusion: Shielding of silver-palladium metal-ceramic restorations with non magnetic material is recommended before exposure to MRI signals.
Keywords: Silver-palladium; Magnetic resonance imaging; Shear bond strength; Dental ceramic
Introduction
Magnetic Resonance Image (MRI) is a non-invasive medical device because it does not use ionizing radiation (X-Ray) [1]. It provides a high resolution image that helps physicians to diagnose and treat medical conditions. MRI is widely used in dentistry as a standard assessment of TMJ disorder and in the oral and maxillofacial field [2-3]. Recently, it is applied for endodontic treatment to obtain the correct and precise topographical images of the root canals and carry out precise mapping of the shape of dental cavities. MRI can be used to visualize the dental surface geometry of decayed teeth and distinguish between soft tissue “pulp” and mineralized tissues enamel, dentine, and root cement [4-5]. Bracher et al. [6] reported that the effectiveness of ultra-shot echo time MRI in caries assessment.
Nowadays different dental alloys have been widely used in fixed prosthodontics, complete denture, removable partial denture and dental implants. Tayamaet et al. [7] suggested that periodical evaluation of these materials using MRI is a very essential method of investigation. Taking MRI images while these alloys exists within human body represent a problem, since they induce heating and magnetic field interactions which lead to artifacts and misrepresentation [8]. Chen et al. [9] reported that metal-ceramic crowns caused moderate artifacts in the MRI and could influence the diagnostic interpretation of MRI locally. There is also possibility of changing the implant orientation, failure of magnets used in over dentures and magnetic keepers and deflection of orthodontic wires are possible. Moreover, minute fracture or crack at surface of the fixed prostheses and dislodgement may happen [10].
Most of previous researches focused on the artifacts happened from dental materials. However, El Bediwi et al. [11-12] studied the effect MRI on the microstructure and physical properties of Ni-Cr, Co-Cr dental alloys and pure dental titanum. The effect of MRI on the shear bond strength of Ni-Cr dental alloy and commercial pure titanium to feldspathic dental porcelain after exposure to MRI signals were also evaluated. There are significant effects were recorded but there is lack of research work in this area.
Metal ceramic prosthesis recorded many advantages during their application for improving clinical performances and mechanical properties. Among the variety of metal ceramic dental alloys, silverpalladium alloys that developed by Heraeus Kulzer in 1931 [13], which contain little or no gold, a minimum of 25% palladium and its properties are similar to type III gold. These alloys offer a suitable alternative to gold alloys and far less costly than gold alloys. On the other hand, they have high sagging resistance during porcelain firing and are very rigid so they are good for long span bridge. They are also easier to solder and easier to work than the base metal alloys [14].
MRI safety and the biocompatibility of dental alloys must be considered before an MRI procedure [15]. Effect of MRI on the microstructure and physical properties of dental materials that have been recently developed and introduced to the dental market were not investigated. It is interesting to evaluate the effect of MRI on the microstructure and physical properties of silver-palladium dental alloys. Moreover, the shear bond strength between silver-palladium alloy and veneering ceramic. Therefore, the aim of this study was to evaluate the effect of MRI signals on the microstructure, mechanical properties and metal ceramic adhesion of commercial silverpalladium alloy. X-ray diffraction pattern analysis, Vickers hardness test, shear bond strength test and Scanning Electron Microscope (SEM) were used for these evaluations. Moreover, hardness of veneered ceramic was also evaluated at the same exposure times.
Materials and Methods
Specimen preparation
Sixty self-cured acrylic resin discs (DuraLay, Reliance Dental Manufacturing Co., Worth, IL, USA), 1 mm in thickness and 15 mm in diameter were prepared in a split cylindrical stainless steel mold. The material was inserted into the mold and covered with a glass plate in order to obtain a flat surface. After complete curing, the discs were removed from the mold, attached to the wax sprue and invested with investment material (Castrate® all speed, Dent aurum, Germany) then burnout in furnace (Vulcan 3-1750, Dentsply, USA). Casting was done using Silver Palladium Universal alloy (BioUniversal® E, IvoclarVivadent, USA). After divesting, the metal specimens were airborne-particle abraded with 110 μm Al2O3 powder (Korox 110, BEGO, Germany) under 30 pounds of air pressure. using (Zhermack®, Technical, S24R, Zhermack Sp, Badia Polesine (RO), Italy). Before ceramic veneering, the metal specimens were once more airborne-particle abraded with 110 μm Al2O3 powder for 10 seconds, at a distance of 2 cm under pressure 60 psi and with an angle of 45 degrees. Then the metal specimens were cleaned ultrasonically in (JELENKO, Germany) with distilled water followed by isopropyl alcohol for 3 minutes each.
Sixty discs were randomly divided into two groups. The first group (N=30) metal discs used for microstructure evaluation, Vickers hardness test and X-ray diffraction analysis. These evaluations were done without exposure to MRI signals as control specimens and after exposure to MRI signals (1.5 T MR scanner) for 15 and 30 minutes. Ceramic veneer was applied to the second group of metal discs (N=30).
Ceramic layer application
Two layers of opaque ceramic (VITA VM®13, VITA Zahnfabrik, Säckingen, Germany) (total thickness: 0.2 mm) were applied with a thin brush onto the air abraded metallic surface by the same operator for standardization. Each layer was fired into furnace (Programat P500/G2, Ivoclar Vivadent AG Bendererstr, Liechtenstein) according to manufacture’`1s direction and the thickness of the opaque layer was carefully measured using a digital caliper (StarrettR 727, Starrett, and Itu, Brazil).
A specially designed split Teflon mold (6 mm in diameter and 4 mm in thickness) was used to apply veneering ceramic onto metal disc, this mold was fabricated with a circular area on one side and the other side has a centralized area for the metal surface to be adapted to create a standardized area for the ceramic application over the center of each disc. Firing of the veneering ceramics was accomplished and second firing was performed to compensate the sintering contraction of the ceramics. The veneered specimens were subjected to 6000 thermocycles in distilled water between 5°C and 55°C then randomly assigned to three groups according to exposure time to MRI nonionizing radio frequency (RF) (1.5 T, Magnetom Vision, Siemens, Germany): a) no MRI exposure (control group), b) 15 minutes of MRI exposure, and c) 30 minutes of MRI exposure.
Ceramic specimen preparation for microhardness
To study the effect of MRI signals on the hardness of veneered ceramic without metal substrate. 30 disc specimens (6 mm in diameter and 4 mm in thickness) were prepared from ceramic (VITA VM®13, VITA Zahnfabrik, and Säckingen, Germany) according to manufacturer’s direction in stainless steel split mold. All specimens were fired in a programmable and calibrated porcelain furnace and the entire specimens were glazed then fired. Specimens were randomly assigned to three groups according to MRI exposure time; no exposure, 15 and 30 minutes.
The tested groups (N=10 each) are distributed as the followings;
Metal (M), Metal-Ceramic (MC) and Ceramic (C)
Group (M1): Control (no exposure to MRI signals).
Group (M2): Specimens were subjected to MRI signals for 15 minutes.
Group (M3): Specimens were subjected to MRI signals for 30 minutes.
Group (MC1): Control (no exposure to MRI signals).
Group (MC2): Specimens were subjected to MRI signals for 15 minutes.
Group (MC3): Specimens were subjected to MRI signals for 30 minutes.
Group (C1): Control (no exposure to MRI signal).
Group (C2): Specimens were subjected to MRI signals for 15 minutes.
Group (C3): Specimens were subjected to MRI signals for 30 minutes.
The direction of the applied magnetic field was normal to the longitudinal direction of the specimen.
X-ray diffraction analysis (XRD)
Microstructure analysis of each specimen was performed on the flat surface of all metal specimens using X–ray diffractometer (Dx–30, Shimadzu, Japan) of Cu–Ka radiation with l=1.54056 Å at 45 kV and 35 mA and Ni–filter in the angular range 2q ranging from 0 to 90° in continuous mode with a scan speed 5 degree/min.
Vickers hardness test
Microhardness test was conducted for each specimens using digital Vickers microhardness (Model FM–7, Tokyo, Japan), with a load of 100 g for 5 seconds via a Vickers diamond pyramid. Each microhardness value quoted is the average of five indentations.
Scanning electron microscope evaluation
The metal specimens were prepared for SEM examination using (JSM-6510 LV, JEOL Ltd, Tokyo, Japan) at 20 kv.
Shear bond strength testing
Each metal ceramic specimen was embedded in self-cured acrylic resin (Acrostone, USA) inside a plastic ring (25 mm in diameter and 20 mm height). Shear bond strength test was carried out at room temperature and performed in a universal testing machine (Lloyd Model TT-B, Instron Corp., Canton, MA, USA). The specimens were mounted in a V-shaped holding device and sheared with a 30° mono beveled chisel edged blade which was aligned 0.1 mm away from the bonded interface. The bonded porcelain specimens were placed under sustained, continuous loading with 25 KN capacities and under a crosshead speed of 0.5 mm/s at 0.5 mm/min until fracture occurred. The shear bond strength (MPa) was calculated by dividing the fracture load (F) in Newton by the surface area (A) in mm2.
Statistical analysis
Statistical analysis was performed using SPSS 11.0 software for windows (SPSS; Chicago, IL, USA). One way analysis of variance (ANOVA) was used to compare the mean of variables at different MRI exposure time. P-values < 0.05 were considered to be statistically significant in all tests.
Results
Shear bond strength
Means and standard deviations of the shear bond strength of the tested specimens were shown in (Table 1), MRI exposure for 30 minutes significantly decreased the shear bond strength of ceramic/ silver-palladium interface (20.74±0.31 MPa) compared to the control specimens (no MRI exposure) (33.51±0.25 MPa).
Groups
N
Mean
±SD
p-value
Control
10
33.51
0.25
<0.0001
15 min
10
28.07
0.39
30 min
10
20.74
0.31
Table 1: Mean (SD) shear bond strength values in MPa of ceramic/silverpalladium alloy specimens before and after MRI exposure.
XRD analysis
Figure 1a-1c shows XRD patterns of silver-palladium dental alloy before and after exposure to MRI for 15 and 30 minutes. The details of formed phases such as; intensity, position and Miller indices of silver-palladium alloy are shown in (Table 2). From X-ray analysis, it is obvious that the silver-palladium alloy consists of Ag-Pd face centered cubic phase. Also formed peaks of silver-palladium alloy representing; the shape intensity, broadness, position and number changed after exposure to MRI. This means that the interaction of MRI with silver-palladium matrix alloy caused changes due to its thermal and magnetic effects.
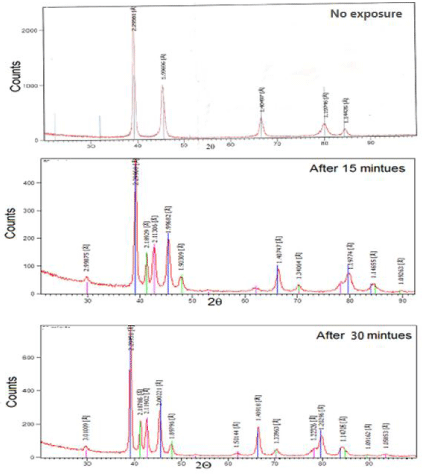
Figure 1: X-ray diffraction patterns of silver-palladium alloy a) before
exposure to MRI (control), b) after 15 min MRI exposure, c) after 30 min MRI
exposure. Changes in peaks shape intensity, broadness and position related
to MRI interaction with silver-palladium matrix.
Silver-palladium specimens (no exposure)
2q
d Å
Int. %
hkl
FWHM
39.2462
2.29561
100.00
100
0.3542
45.4186
1.99696
39.98
200
0.3149
66.5586
1.40497
14.14
220
0.7872
80.1531
1.19746
9.07
311
0.8659
84.6265
1.14426
4.74
222
0.9600
Table 2: X-ray diffraction analysis of silver-palladium alloy before and after MRI exposure.
After exposure for 15 minutes
2q
d Å
Int. %
hkl
FWHM
29.7944
2.99875
4.3
0.4723
39.1738
2.29968
100
100
0.3936
41.2365
2.18929
24.3
0.2362
42.796
2.11306
29.24
0.5904
45.422
1.99682
37.22
200
0.551
47.7944
1.90309
8.84
0.6298
66.425
1.40747
16.91
220
0.4723
70.2066
1.34064
4.83
0.7872
80.1305
1.19774
10.84
311
0.9446
84.5048
1.14655
5.11
222
0.7872
89.6584
1.09263
0.95
1.152
After exposure for 30 minutes
2q
d Å
Int. %
hkl
FWHM
29.6797
3.01009
3.2
0.6298
39.1757
2.29958
100
100
0.3936
41.2648
2.18786
24.11
0.2558
42.6696
2.11902
25.46
0.551
45.2928
2.00221
32.7
200
0.2755
47.9319
1.89796
7.48
0.7085
61.79
1.50144
1.6
0.09
66.3339
1.40918
22.32
220
0.2558
70.2674
1.33963
4.31
0.7872
78.1344
1.22326
6.35
1.1195
79.7133
1.20296
14.76
311
0.8659
84.4596
1.14705
5.8
222
0.9446
89.764
1.09162
0.67
1.152
93.49
1.05853
0.93
0.09
Table 2 of 2:
Vickers hardness of silver-palladium alloy
The hardness of silver-palladium alloy was conducted using a digital Vickers hardness tester, applying a load of 100 g for 5 seconds. The hardness was significantly decreased after 30 minutes exposure to MRI compared to control group (334.66±0.28 and 233.13±0.36), respectively as shown in (Table 3).
Groups
N
Mean
±SD
p-value
Control
10
334.66
0.28
<0.0001
15 min
10
262.02
0.25
30 min
10
33.13
0.36
Table 3: Mean (SD) Vickers hardness values of silver-palladium alloy before and after MRI exposure.
Scanning electron microscope evaluation
Scanning electron micrographs of silver-palladium alloy as a control and after exposure to 1.5 T MRI for 15 and 30 minutes are shown in Figure 2a-2c. SEM analysis of silver-palladium alloy showed a considerable change of the microstructure after exposure to MRI (Figure 2a), lamellar structure with fine grain and different rod shape was detected. Figure 2b-2c showed a change in lamellar structure and large grains with different orientations and formed different slabs). These results were also proved by the X-ray diffraction analysis. Fine dispersions were observed at control group (M1) and it was changed to coarse grains after MRI exposure time at 15 and 30 minutes in groups (M2 and M3).

Figure 2: SEM of silver-palladium alloy a) before exposure to MRI (control),
b) after 15 min MRI exposure, c) after 30 min MRI exposure. Considerable
microstructure changes in the surface after MRI exposure.
Vickers hardness of veneered ceramic
Figure 3 and (Table 4) showed that the Vickers’s hardness of feldspathic ceramic was decreased with increasing the MRI exposure time compared to control specimens. There was significant decrease in hardness after exposure to MRI from 562.20±1.25 to 409.4±0.43.
Groups
N
Mean
±SD
p-value
Control
15 min
30 min
10
10
10
562.20
459.30
409.40
1.25
1.00
0.43
<0.0001
Table 4: Mean (SD) Vickers hardness values of ceramic specimens before and after MRI exposure.
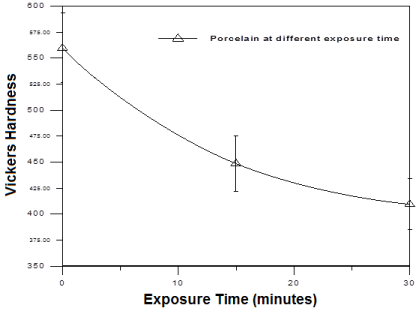
Figure 3: Vickers hardness of the ceramic specimens before and after
MRI exposure. It showed continuous decrease in ceramic hardness with
increasing exposure time to MRI.
Discussion
Conventional porcelain fused to metal restorations demonstrated superior fracture resistance due to supportive metal substrate; however, fracture and chipping can be reported. Silver-palladium alloy used in this study is a common alloy used as metal coping which have high resistance to sagging during porcelain firing and are very rigid. They are suitable for long span prosthesis and more liquefy in the molten state, easier to solder, and easier to work with than the base-metal alloys. Obtaining successful restorations are depending on the strength of bonding. These restorations are subjecting to failures that could occur predominantly at the interface between the metal and the porcelain. Retention of porcelain to metal can be obtained micromechanically or chemically or both. In our study, the retentive means was micromechanically using air-abrasion with 110 μm Al2O3.
In general hardness is defined as resistance of material to plastic deformation caused by the impact of a harder test object, usually by indentation. Hardness may also refer to stiffness or resistance to scratching abrasion, or cutting.
The hardness of dental alloys is usually determined using the Vickers hardness test (DIN 50133). In this test, a pyramid-shaped diamond point is pressed against the polished surface of the alloy at a defined force to create an indentation. The hardness of an alloy is one important criterion for its abrasion resistance. The hardness of an alloy is usually correlated with mechanical strength. The hardness significantly decreased with increasing the MRI exposure time. These changes in the alloy matrix microstructure may be attributed to the MRI thermal and magnetic effects that influence the bond strength by reducing its hardness. These results coincided with previous results reported the hardness of Ni-Cr and titanium alloy were decreased with increasing the MRI exposure time [12]. X-ray diffraction crystallography is widely employed to ascertain relative arrangement of atoms in metals. It can determine the phase transformation and composition of materials. Moreover, study internal stress in lattice and grain size [16]. Therefore, it was used in this study and its data supported the results obtained from Vickers hardness of the Ag-Pd alloy and is coherent with the observed microstructure.
MRI uses a magnetic field and pulses of radio-wave energy to produce detailed pictures of organs, and structure inside the body and it may give information about structures problems more clearly than other investigation methods and the resulting images can be examined on a computer monitor [2]. Previous data suggest that there are no adverse effects caused by short term exposure of head to static magnetic fields up to “2” tesla (T) “ each tesla = 10.000 of earth gravity” and the exposure of the whole body should not exceed “4T” which considered high Telsa device [11,17]. In this study, it was obvious that the effect of MRI signals exposure time on the hardness of the veneered ceramic was decreased significantly with increasing the exposure time from (562.20±1.25) in M1 control group (no exposure time) to (409.4±0.43) in M3 group 30 minutes exposure. Silver-palladium alloy consists of Ag-Pd face centered cubic phase. The shape and the number of formed phases (intensity, broadness and position) changed after exposure to MRI.
Several tests were reported in the literature to evaluate the metalceramic bond strength, such as twist, shear, tension, flexural mode all showing advantages and disadvantages [18]. The dominant stress in the shear bond test is shear stress, so it is recommended to measure bond between two materials [3,19,20]. Oral fluids are known to cause stress corrosion on the ceramic surfaces or at the interfaces resulting in slow crack growth [21]. Testing the adhesive joints either after water storage or thermocycle yield to hydrolytic degradation at the interface and usually results in decreased bond strength at metal/ ceramic interface [22,23]. Therefore, in the present study shear bond was tested using the universal testing machine after subjecting the specimens to 6000 thermocycles.
The success of a metal-ceramic restoration function and esthetics depends primarily on strong adhesion between the veneering porcelain and metal coping interface. In this study we studied the effect of MRI signals at different exposure time on the surface properties of silver-palladium alloy and bond strength of metal/ceramic interface. Moreover, shear bond strength of the tested specimens showed lower values with increasing the MRI exposure time. These changes may be attributed to the magnetic effect of MRI that cause changes in H-O bond (orientations/ or break) as see in FTIR analysis of ceramic materials. Also matrix structure of ceramic and alloy effected due to thermal and magnetic effects of MRI. Other studies stated that shear bond strength values greater than 10 MPa indicate clinically satisfactory results and representing a better bond strength than the necessary to provoke the clinical flaw of union between metal and ceramic [20,24-26]. Certainly, it would be interested to study surface roughness, corrosion resistance and effect of cycling loading on the bond strength of metal/ceramic interface after MRI exposure, which considered limitations of this study.
Conclusions and Recommendations
Within the limitations of this study, we can conclude the followings:
1. Shear bond strength of silver-palladium/ceramic interface was decreased after exposure to MRI radiation, however, it was within the acceptable range.
2. Vickers hardness number of silver-palladium alloy and ceramic veneer was decreased after exposure to MRI signals.
3. Shielding of silver-palladium metal-ceramic restorations with non magnetic material is recommended before exposure to MRI radiations.
4. Due to the limited studies investigated the effect of MRI radiation on the different dental restorative materials, therefore, future studies are required.
Acknowledgements
This research was supported by Mansoura University, Mansoura, Egypt.
References
- Taqa A, Al-Noori A, Al-Khyaat A. The effect of Magnetic Resonance Imaging (MRI) on some properties of acrylic resin denture base materials. American Journal of Medical Sciences and Medicine. 2013; 1: 62-65.
- Edwards BM, Taylor MK, Shellack GF. Prosthetic heat values, evaluation of magnetic field integration at'' 1.5'' tesla. J Magnet Resona Imag. 2000; 12: 363-369.
- Akova T, Ucar Y, Tukay A, Balkaya MC, Brantley WA. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent Mater. 2008; 24: 1400-1404.
- Farina D, Bodin C, Eanclolfi S, Gasperi DW, Borghesi A, Maroldi R. TMJ disorders and pain assessment by contrast-enhanced MRI. Euro J of Radio. 2009; 70: 25-30.
- Tanasiewicz M. Magnetic resonance imaging in endodontic treatment predication. J Med Imag and Radio Scien. 2010; 41: 127-132.
- Bacher AK, Hofmann C, Bornstedt A, Rasche V, Hell E, Haller B. Ultra shot echo time (UTE) MRI for the assessment of caries lesions. Dento maxillofac Radiol. 2013; 42.
- Tayama S, Kunieda E, Takeda T. Stereotactic radio-surgery with an immediate partial denture. Kio J Med. 2008; 5: 111-123.
- Hideshima M, Mizutani H, Ando T, Destine D, Ishika S, Matsuzaki S, et al. Effects of dental alloys and magnetic keeper on MRI: relationship between cast crown and artifacts of axial plane Image. J Magnet Resonan Imag. 2011; 5: 1-7.
- Chen D, Wu G, Wang Y. Influence of galvano-ceramic and metal-ceramic crowns on magnetic resonance imaging. J Chin Med. 2010; 123: 208-211.
- Bagheri H, Hosseini M, Emami J, Forough A. Metallic artifact in "MRI" after removal of implants. Europe. J Radio. 2010; 20: 1016-1020.
- El-Bediwi A, Mohamed S, El-Fallal A, El-Khaligy S. Influence of gamma radiation and magnetic resonance imaging radiation on micro-structure, hardness and electrochemical corrosion behavior of Co?Cr-based dental alloy. Radiation Effects & Defects in Solids. 2011;166: 223-227.
- El-Bediwi A, El-Fallal A, Saker S, Ozcan M. Effect of non-ionizing radio frequency Signals of magnetic resonance imaging on physical properties of dental alloys and metal-ceramic adhesion. J Adhes Dent 2014; 16: 407-413.
- Heraeus Group.
- John F McCabe, Angus WG Walls. Applied Dental Materials 9th edition.2008.
- Hubalk H, La Serna P, Linetskiy I, Dostalova T. Dental alloys and magnetic resonance imaging. Int Dent J. 2006; 56: 135-141.
- Nasel CJ, Pretterklieber M, Gahleitner A, Czerny C, Breitenseher M. Imh of Osteometry of the mandible performed using dental MR imaging. AJNR Am J Neuro radiol H. 1999; 20: 1221-1227.
- Kansu G, Aydin AK. Evaluation of the biocompatibility of various dental alloys: Part 2–Allergenical potentials. Eur J Prosthodont Restor Dent. 1996; 4: 155-161.
- Joias RM, Tango RN, Junho de Araujo JE, Junho de Araujo MA, Ferreira Anzaloni Saavedra Gde S. Shear bond strength of a ceramic to Co-Cr alloys. J Prosthet Dent. 2008; 99: 54-59.
- Papazoglou E, Brantley WA, Johnston WM, Carr AB. Effects of dental laboratory processing variables and in vitro testing medium on the porcelain adherence of high-palladium casting alloys. J Prosthet Dent. 1998;79: 514-519.
- Kohorst P, Dittmer MP, Borchers L, Stiesch-Scholz M. Influence of cyclic fatigue in water on the load-bearing capacity of dental bridges made of zirconia. Acta Biomater. 2008; 4: 1440-1447.
- Özcan M, Nijhuis H, Valandro LF. Effect of various surface conditioning methods on the adhesion of dual-cure resin cement with MDP functional monomer to zirconia after thermal aging. Dent Mater J. 2008; 27: 99-104.
- Özcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cements to ceramics. Dent Mater. 2003; 19: 725-731.
- Anusavice KJ. Noble metal alloys for metal-ceramic restorations. Dent Clin N Amer. 1985; 29: 789-803.
- Hammad IA, Talic YF.Designs of bond strength tests for metal-ceramic complexes: review of the literature. J Prosthet Dent. 1996; 75: 602-608.
- Poljak-Guberina R, Catovic A, Jerolimov V, Franz M, Bergman V.The fatigue strength of the interface between Ag-Pd alloy and hydrothermal ceramic. Dent Mater. 1999; 15: 417-420.