
Research Article
J Dent App. 2022; 8(1): 472-476.
Alumina: As a Biocompatible Biomaterial Used in Dental Implants
Kavya K1*, Sharanraj V2*, C.M. Ramesh3, Naveen Kumar M4, Vasantha Kumar5, Sadashiva M6, T Anil Kumar7, Chandan B R8
1Assistant Professor, Department of Zoology, St. Joseph’s College (Autonomous), Langford Road, Bengaluru - 560027, Karnataka, India
2Senior Grade Lecturer, Department of Mechanical Engineering (W&SM), Sri Jayachamarajendra (Govt.) Polytechnic, K.R Circle, Bengaluru - 560001, Karnataka, India
3Associate Professor, Department of Mechanical Engineering, Ramaiah Institute of Technology, Visvesvaraya Technological University, Bengaluru - 560054, Karnataka, India
4Assistant Professor, Department of Biotechnology and Genetics, Ramaiah College of Arts, Science and Commerce, Bengaluru - 560054, Karnataka, India
5Associate Professor, Department of Mechanical Engineering, Bearys Institute of Technology, Visvesvaraya Technological University, Mangalore - 574153, Karnataka, India
6Assistant Professor, Department of Mechanical Engineering, PESCE, Visvesvaraya Technological University, Mandya – 571422, Karnataka, India
7Associate Professor, Department of Mechanical Engineering, Ramaiah Institute of Technology, Visvesvaraya Technological University, Bengaluru -560054, Karnataka, India
8Professor & Head, Department of Mechanical Engineering, G. Madegowda Institute of Technology, Visvesvaraya Technological University, Mandya – 571422, Karnataka, India
*Corresponding author: Kavya K, Assistant Professor, Department of Zoology, St. Joseph’s College (Autonomous), Langford Road, Bengaluru - 560027, Karnataka, India
Sharanraj V, Senior Grade Lecturer, Department of Mechanical Engineering (W&SM), Sri Jayachamarajendra (Govt.) Polytechnic, K.R Circle, Bengaluru - 560001, Karnataka, India
Received: July 06, 2022; Accepted: August 01, 2022; Published: August 08, 2022
Abstract
Alumina is a ceramic bio inert biomaterial having purity 99.5% Aluminium oxide (Al2O3) and 0.5% Magnesium oxide (MgO), find its wide application in dental implant due to good bio-compatible property with adjacent tissue, better wear and aesthetic characteristics. This paper represents the in-vitro tests conducted to evaluation toxicity by cell culturing on Alumina biomaterial used in the dental implant by both direct contact and extraction method. In the present study in-vitro assessment of tissue bio compatibility was conducted on L929 cell line (mouse fibroblast). In-vitro test, the toxicity of Alumina specimen was done by computing percentage of viability in a cell cultured medium. An MTT system was used to measure the active cell activities with mitochondrial-dehydrogenases, which is an easy method which gives accurate and precision results. The results of biocompatibility in-vitro test by both Direct and Extraction methods confirmed that Alumina exhibits a highest cell growth of 93.05% and resulted with zero grade cytotoxicity. Alumina having good aesthetic characteristics i.e., colour of the implant matches with the tooth colour. Hence Alumina is a best candidate alternate implant material compared to other metal implants.
Keywords: Alumina (Al2O3); Bioinert material; Alternate dental implant; Cytotoxicity; MTT
Introduction
Alumina is traditionally referred as aluminium oxide, which is used as a bio-inert material. Aluminum oxide is the principal oxide of aluminium. Alumina forms the basis of a very wide and important range of ceramic articles and components. Its great usefulness as a material has resistance to hardness, withstand higher temperatures, less electric conductivity. Alumina is the major constituents have high density hence forms a high grade refractory material. As micrometer grain size alumina material, it provides good wear resistance and insulating property. Alumina do not induce direct bond with the host tissue, instead they are encapsulated by a characteristics thin layer of fibrous tissue after implantation.
Alumina having purity greater than 99.5% find its importance in implant application due to good bio-compatible property with adjacent tissue, better wear and friction property and Aesthetic characteristics. Alumina has less tensile strength due to its brittle nature of the material. Alumina with high density and higher purity has good corrosion resistant bio-compatible better compression strength and good wear resistant making it as a useful ceramic single piece implant [1].
Alumina Refining Process
The oxide of alumina is the third most common element in the earth’s crust, but it exists primarily in the form of bauxite ore. The most common mineral constituent of bauxite is gibbsite but the boehmite and diasporeoxy hydroxide phases are also found. Pure single crystal aluminum oxide is colorless and transmitting radiation over a wide spectral range. (Figure 1) shows the flow chart of alumina refining process [2].
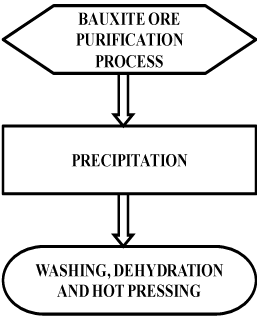
Figure 1: Alumina specimen as per ASTM E384standard specification.

Figure 1b: Alumina specimen as per ASTM E384standard specification.
Bauxite contains aluminum oxide and oxide impurities like Fe2O3, SiO2, Ga2O3 and TiO2. Bauxite ore is first crushed and purification is achieved through process of diluting bauxite in powder form with sodium hydroxide treated at 300oC under normal pressure. The above solution is later treated from normal temperature to super saturated temperature of sodium aluminate. Excess oxides are removed by seeding technique, where gibbsite is obtained by precipitating aluminum oxide. Once, precipitated material is washed and then dehydrated at temperature of 1000oC to 1200oC to convert low temperature forms alumina, a coarsely crystalline material within which large single crystals can be formed [3].
The bulk fusion and cooling of alumina powder yields fused alumina or aluminum oxide having hexagonal closed packed crystalline structure. This aluminum oxide powder is hot pressed at a pressure of 35 – 70 MPa at temperature of 1200oC – 1400oC using graphite dies to desired shape and size. The melting point of alumina is 2072oC and modulus of elasticity is 318 GPa [4]. Alumina is a single piece implant when compared to other two piece implant.
Application of Alumina
The following are the application of alumina
• Load bearing hip
• Knee prostheses
• Alveolar ridges
• Dental crown
• Dental screws
• Cardiovascular devices
Limitations of Alumina
The limitation of alumina is as follow
• Alumina is a brittle material hence; chances of fractures are more [5].
Biocompatibility
Biocompatible is measure of biomaterial ability to adhere to the biological condition to body. To determine biocompatible property, various biocompatibility tests are conducted and observations are made to evaluate the toxic effects of these materials when implanted in body. Typically material testing and analysis of a biomaterial component are conducted prior to any biological testing to check their cytotoxicity [6].
Cytotoxicity measures an ability of biomaterial to destroy living cell. Biocompatibility tests are categorized into two categories namely in vivo test and in vitro test. In invivo test experiments are conducted directly on the living cells or organisms usually humans and animals. In in-vitro test experiments are performed in test tube or outside living organisms. Once in vitro testing has been completed and satisfied, only then in-vivo testing will be performed to cross verify that the biomaterial will serve the purpose and that is to test biological response like irritation and other clinical testing.
The biomaterials undergo the following different kinds of tissue reactions:
1. Toxic: tissue cells death occurs
2. Nontoxic and inactive biological nature: fibrous growth between tissues.
3. Nontoxic and biologically active: bonding between tissues occur
4. Nontoxic and dissolve: replacement of adjacent tissue [7].
In-vitro test are conducted to evaluate whether tissue is biocompatible or not. These tests are done by cell culture method which is very effective method applied in medical field to evaluate diverse materials in medical industries [8].
Types of Cell Culturing Methods
The various types of cell culturing methods are:
1. Direct-contact method
2. Agar-diffusion method
3. Extraction method
For conducting the above mentioned test some of the variables of the experiment must be constantly monitored. Few of these constants are L929 cell line (mouse fibroblast), cell number, exposure time, sample size. The viability test was calculated comparing with control. The quantity of the cell death by viability test gives the measurement of cytotoxicity and in turn measures the biocompatible property of biomaterial tested.
Direct contact method: The test specimen is located directly on the cell line cultured medium. In this method blue haematoxylin is added to dilute the medium. Cytotoxicity is measured by the percentage of cell inhibition where death cells does not take up the stain and percentage of viability measures the stained cells which gives cell growth percentage.
Agar diffusion method: an alga made up of polymer is applied between the cells and sample. During this test the cytotoxicity is measured by comparing the active cells with death cells. Active cells take up stain which is red in colour [9].
Extraction process: Process of elution, in which the leachates from the contact bio-material is extracted and treated, assessment will be made directly on the human cells and medium by implanting it and check for any harmful effect in the potential behaviour and whether the bio-material is biocompatible with the adjacent tissue by surgical method [10]. The dimensions and geometry of implant plays a vital role after surgery, the material reaction with the corresponding adjacent tissue is monitored. So that any foreign particles present in implants may cause harmful toxic effect to the cell [11].
An ideal condition for implantation means implant must be easily and safely integrated to the surrounding adjacent tissues with very minimum time and the wound should heal as fast as possible and restore the damage one and replace it. But in real condition, now a day’s the implantation done under normal condition has lead to the various harmful effect due to surrounding adjacent tissue problems like tissue over growth, improper bonding of implant with tissue, implant fracture inside and in certain serious problems implant may have to be removed from the body completely. The duration of time for the implantation should be as minimum time as possible [12].
To prevent implant failure, standard clinical procedure has to be followed. Only stable material should be chosen for implantation, if the unstable material is chosen more chances of implant failure occurs [13]. Stable material will not react with the adjacent tissue easily and will have minimum toxic effect on the body, enhances biological properties and are highly biocompatible. While designing implant Computer modelling and FEA should be carried out along with simulation for better understanding by advanced simulating tool to identify the peak stress and strain values that cause failure of implant. After fabrication of implant, both mechanical test and biological test should be performed to recommend it for clinical application or intended purpose [14].
In the present study in-vitro assessment of tissue bio compatibility was conducted on L929 cell line (mouse fibroblast) by direct contact test and agar diffusion test method. In-vitro test, the toxicity of specimen has been done by computing percentage of viability in a cell cultured medium. Alternative process is to calculate radio-isotope incorporated in DNA by counting the automated counters and other related activities of cell. An MTT system means measuring the active cell activities with mitochondrial-dehydrogenases. It is easy method which gives accurate and precision results [15].
A MTT is [3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] an important substance made up of soluble water salt resulting in yellow liquid solution prepared in a media. MTT at this stage is converted to formazan soluble solution by enzyme (mitochondrial-dehydrogenases) of cell viability. This formazan solution is solubilised by DMSO solvent to convert from yellow to purple solution and it is measured spectrophotometrically depending on cytotoxic effect induced by the test specimen, the cell number may reduce or enhances. Hence, this leads to measure cytotoxicity [16,17].
There are certain criteria for selection of implant and implant should posses following:
• Biocompatible in nature Material must be bio-inert
• Good aesthetic property.
• Free from allergy
• Corrosion resistant and
• Wear resistant
• Economical
• Easily manufactured.
Materials and Methods
Material Preparation
Alumina block was sintered at a temperature of 1500oC for 1 Hr as per ASTM E384 standard specification and 20% of volumetric shrinkage was observed.
The Alumina specimen as per ASTM E384standard specification is shown in FIG 1.
TAB 1 shows the Dimensions of Alumina specimen prepared for biocompatible test.
Biocompatibility Testing Methods
The purpose of biocompatibility test or assay is to assess the effects of the leachates from the Aluminaon the L-929 mouse fibroblast cell line. For conducting biocompatibility test CO2 incubator, P35 dishes, Autoclave, Test tube, Cell culture reagents such as DMEM, FBS, Pen strip and Trypsin were used. L929 mouse fibroblast cell line, treated in supplement of DMEM with Fetal Bovine Serum 10% in inactive condition, 100 IU/ml quantity of penicillin, 100 μg/ml streptomycin, 5 μg/ml of amphotericin with 5% CO2 was humidified at 37oC temperature atmosphere till it reaches confluent stage. TPVG solution which contains 0.2 % trypsin, 0.02 % EDTA, 0.05 % glucose in PBS was used to cell dissociation. Checking was done for cell viability. On other hand 10, 00, 000 cells and 50,000 cells of L-929 fibroblast cell line was seeded in a 96 well plated P35 dish respectively and incubation was carried out at temperature 37oC with CO2 5% incubation for 24 hours as per ISO:10993-5 standard [12].
This research paper presents two methods of biocompatibility tests were carried out namely Direct Contact method and Extraction method.
Direct Contact Method: In Direct contact method sample specimen was cut and placed at the center of a cultured dish and incubated for 24 hours. Post incubation was carried out in which, the samples were removed carefully and cell morphology was observed under a microscope and scored according to the (Table 2.1).
Dimensions
Before Sintering
After Sintering at 1500 OC
Volumetric Shrinkage
Breadth(mm)
Depth(mm)
Length (mm)
Breadth (mm)
Depth (mm)
Length (mm)
Percentage (%)
15
15
20
12
12
16
20
Table 1: Dimensions of Alumina specimen.
Grades
Reaction level
Reaction Zone description
0
None
No cell death zone
1
Slight
Only Some cell death zone under specimen
2
Mild
Few cell death with limited zone to area below specimen
3
Moderate
Cell death zone extending specimen size up to 10mm
4
Severe
Cell death zone beyond the specimen more than 10mm
Table 2.1: Reactivity grades for Direct Contact Test [12].
Extraction Method: In Extraction Method, Leachates from the samples were suspended in a DMEM plain media and 100μl was added to the well containing L929 mouse fibroblast cells in a 96 well plate and incubated for 24hrs by adding 100 μl of MTT to all well. Separately, test solutions were discarded in each well after the process incubation [13,14]. Incubation was done for 4hrs in CO2 content 5% atmosphere at 37oC. Removal of supernatant is done by adding DMSO of 100 μl quantity on plates and plate was solubilised by shaking gently to form Formosan. Micro plate reader having 590 nm wave lengths is used to measure absorbance by applying formula the viability and inhibition percentage was calculated. Later morphology grades indicating cytotoxicity in extract, reaction was given according to the (Table 2.2).
Grades
Reaction level
All cell culture conditions and cytotoxic effect
0
None
No cell death or Cell growth unaffected
1
Slight
20% of Cell death occurs and slightly cell growth effected.
2
Mild
50% of Cell death in the medium and growth of cell is effected
3
Moderate
70 % of Cell death but completely not destroyed and growth of cell is effected
4
Severe
Cells are completely destroyed and cytotoxic.
Table 2.2: Morphology grades indicating cytotoxicity reaction in extract [12].
Percentage of Viability and Inhibition is computed by formula [12]:
% of Inhibition = 100 – (outer diameter of sample) x 100
% of Viability = 100 – % Inhibition.
Results and Discussion
Biocompatibility In-Vitro Test Results of different methods are discussed below:
Figure 3.1 shows the microscopic image of a control and direct contact with Alumina bio material. In Figure 3.1(a) control which is an L-929 mouse fibroblast cell cultured in medium microscopic image and Figure 3.2 (b) microscopic image of Alumina biomaterial when place directly on the cultured L-929 mouse fibroblast cell medium. From the above microscopic observation reactivity grade is 1, which has only some cell death zone under Alumina treated specimen.
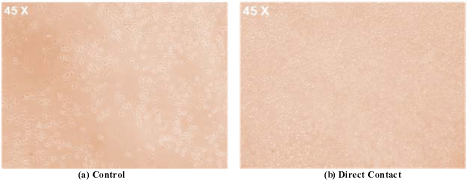
Figure 3.1: Microscopic image of Alumina (a) Control and (b) Direct contact.
Table 3.1 shows the reactivity grade for direct contact test method for Alumina. Direct contact test method result showed that Alumina biomaterial have grade 1 and slight reaction level and only some cell death zone under specimen, which is within recommended limit. Hence Aluminais a best candidate alternate material for metal free dental implant.
Sample
Grade
Reaction level
Figure No.
Result
Alumina
1
Slight
3.1(b)
Only some cell death zone under specimen
Table 3.1: Reactivity level grade for Direct Contact test.
Figure 3.2 shows the microscopic image of a control and Extract leachates Alumina biomaterial is treated. In Figure 3.2 (a) control which is an L-929 mouse fibroblast cell cultured in medium microscopic image and Figure 3.2 (b) microscopic image of Alumina biomaterial leachates were suspended in a cultured L-929 mouse fibroblast cell medium.
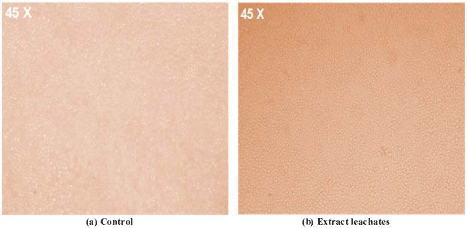
Figure 3.2: Microscopic image of Alumina (a) Control and (b) Extraction
leachates.
Table 3.2 shows the evaluation of effect of leachates on cell viability in L-929 cells where the Alumina cell growth is higher i.e., 93.05 %compared to metal other implants.
Effect of leachates on cell viability in L-929 mouse fibroblast cells
Compound Name
Dilutions in %
OD at 590nm
% Inhibition
% Viability
Result
Control
0
0.7471
0.00
100.00
Cell growth is 93.05 % and Cell death is 6.95%
Alumina
50
0.5392
27.83
72.17
25
0.5597
25.08
74.92
12.5
0.5808
22.26
77.74
6.25
0.6614
11.47
88.53
3.125
0.6795
9.05
90.95
1.562
0.74
6.95
93.05
Table 3.2: Evaluation of effect of leachates on cell viability in L-929 cells.
Table 3.3 shows the Morphology grades indicating cytotoxicity reaction in extract, where Alumina exhibits 0 grade which results in No cell death or Cell growth unaffected. Hence Alumina can be considered as an alternate material to metal dental implant.
Biomaterial
Grade
Figure No.
Result
Alumina
0
3.2(b)
No cell death or Cell growth unaffected
Table 3.3: Morphology grades indicating cytotoxicity reaction in extract.
Conclusions
Biocompatibility in-vitro test conducted by both Direct and Extraction methods, confirmed that Alumina exhibits a highest cell growth of 93.05% and resulted with zero grade cytotoxicity. Alumina having good aesthetic characteristics i.e., colour of the implant matches with the tooth colour. Hence, Alumina having high cell growth percentage can be used as alternate biomaterial dental implant and other medical applications.
References
- D. F. Williams, Definitions in Biomaterials, Proceeding of a consensus conference of the European society of Biomaterials. Elsevier. New York. 1987; 4.
- Amogh Tathe, Mangesh Ghodke, Anna Pratima Nikalje. A brief review: Biomaterials and their applications. International journal of pharmacy and pharmaceutical sciences. 2010; 2: 19-23.
- Buddy D. Ratner, Biomaterials Science: An Introduction to Materials in Medicine. Elsevier Publishers. Amsterdam, 2004.
- Soumya Nag and Rajarshi Banerjee, Fundamentals of medical implant materials, Materials for medical devices. ASM Handbook. 2012; 23: 06 - 07.
- Meleigy EI, Emad, Van Noort, Richard. Selection criteria of ceramics for medical applications. 2012; 19-36.
- DH Barnes, A Moavenian, A Sharma, SM Best. Biocompatibility of ceramics. ASM Handbook. 2012; 23: 128-134.
- Kawahara H, Hirabayashi M, Shikita T. Single crystal alumina for dental implants and bone screws. Journal of biomedical materials research. 1980; 14: 597-605.
- V Sharanraj, CM Ramesha, V Kumar, M Sadashiva. Finite Element Analysis of Zirconia Ceramic Biomaterials Used in Medical Dental Implants. Interceram. Springer. 2019; 68: 24-31.
- J. Black, Biological performance of materials: Fundamentals of Biocompatibilty. 3rd Edition. 1999: 137.
- BD Rather, AS Hoffman, FJ Scheon, JE Lemons. Biomaterials Science: An Introduction to Materilas in Medice, 2nd ed. Elsevier Academic Press, San Diego. CA 2004.
- KC Dee, DA Puleo, R Bizios. An Introduction tp Tissue – Biomaterial Interaction. John Wiley and Sons, Inc., Hoboken, NJ, 2002.
- DW Hoeppner, V Chandrasekaran. Fretting in medical Implant: A Review. Wear. 1994; 173: 189 -197.
- J Cohen. Metal Implants – Historical Backround and Biological Reponse to Implantaion. Biomaterial Reconstruction Surgery. 1979; 46 – 61.
- SG Steinemann. Corrosion of surgical implants-In-vivo and In-vitro Tests Evaluation Biomaterials, 1980.
- Laxman S Desai. Biological Evaluation of Medical Devices —Part 5: Tests for in vitro cytotoxicity and Japanese guidelines; ISO 10993-5.
- Kavya K, Kumar MN, Patil RH, Hegde SM, Kumar KMK, Nagesh R, et al. Differential expression of AP-1 transcription factors in human prostate LNCaP and PC-3 cells: role of Fra-1 in transition to CRPC status. Molecular and Cellular Biochemistry. 2017; 433: 13-26.
- Kavya K, Naveen Kumar Mallesh, Srikantaradhya Chidananda Sharma, Doddamane Manjulakumari. Midostaurin inhibits hormone-refractory prostate cancer PC-3 cells by modulating nPKCs and AP-1 transcription factors and their target genes involved in cell cycle. Front Biol. 2017; 421-429.