
Case Series
J Dent & Oral Disord. 2025; 11(1): 1189.
Fluorescence Enhanced Theragnosis: A Principle that Serves to Educate and Provide Quality Assessment in Clinical Practice and Lab Training – Part A – Clinical Practice
Gal Hiltch¹, Cauana Oliva Tavares¹, José Antonio Poli de Figueiredo¹*, Silvia Dias de Oliveira², Livia Ramos Alvariza¹, Daiane Giron Granzzoto¹ and Liviu Steier³
¹Federal University of Rio Grande do Sul – UFRGS – Graduate Program in Dentistry, Porto Alegre, Brazil
²Pontifical Catholic University of Rio Grande do Sul – PUCRS – Graduate Program in Dentistry, Porto Alegre, Brazil
³University of Pennsylvania – UPENN - School of Dental Medicine, Department of Preventive and Restorative Sciences, USA
*Corresponding author: José Antonio Poli de Figueiredo, Professor in Oral Biology and Endodontology – UFRGS Federal University of Rio Grande do Sul, Rua Sarmento Leite, 500 – room 134, CEP 90050-170 Porto Alegre, RS, Brazil Email: poli.figueiredo@outlook.com
Received: February 22, 2025; Accepted: March 20, 2025; Published: March 24, 2025;
Abstract
Objectives: This study aims to convey the educational possibilities of Fluorescence Enhanced Theragnosis (FET) in the Dental field. Early detection, diagnosis, and prediction of pathological activity are fundamental challenges in contemporary dentistry. Fluorescence-based diagnostic tools have emerged as valuable adjuncts in addressing these challenges. Human tissues and bacteria exhibit distinct bioluminescent properties, emitting different wavelengths and, consequently, producing distinct characteristic colour emissions under certain light conditions.
Materials and Methods: The authors present their clinical experience with the Reveal™ device (Designs For Vision Inc, NYC, New York, USA), which integrates 405 nm and 450 nm LED light sources into hands-free loupes, and demonstrates enhanced diagnostic capabilities and treatment decision-making. This configuration enables more conservative approaches and minimally invasive treatments tailored to each case. This paper presents the theragnosis concept through bioluminescence-based techniques in the oral cavity, supported by clinical cases that demonstrate this approach’s efficacy.
Results: Our findings establish fluorescence as both an educational tool and a clinical aid, enhancing the visualization, diagnosis, and treatment of diverse oral conditions through real-time assessment of pathological processes.
Conclusions: This phenomenon may provide dental students at all educational levels with an effective tool for identifying and understanding various oral conditions, including dental biofilm, white lesions, caries, periodontal disease, peri-implantitis, oral infections, potentially malignant lesions, and oral cancer.
Clinical Relevance: The implementation of Fluorescence Enhanced Theragnosis at early stages, particularly during undergraduate and graduate learning, may result in professionals with more precise diagnostic skills and able to provide minimally invasive interventions with outcomes that are adequate to patients´ needs.
Keywords: Dental biofilm; White lesions; Periodontal disease; Peri-implantitis; Oral infections; Oral cancer
Introduction
A conservative approach in dentistry relies on detecting and resolving pathological threats through minimally invasive and optimal treatment techniques while maintaining tissue balance [1]. Successful conservative dentistry relies on enhanced clinical decisionmaking capabilities supported by accurate diagnostic tools. Recently, non-invasive fluorescence-based diagnostic adjuncts have gained significant recognition for their contribution to diagnostic accuracy [2-9]. Fluorescence occurs when molecules absorb high-energy photons at shorter wavelengths and subsequently re-emit lower-energy photons at longer wavelengths, causing the surface containing these molecules to emit light or "luminescence" [10]. Both human tissues and bacteria possess natural biofluorescent properties, producing characteristic colors when exposed to an external blue light source. In the context of oral bacteria, this fluorescence primarily results from the excitation of specific molecules called porphyrins [11]. As the natural concentration of fluorescent molecules (endogenous fluorophores) varies among different bacteria and human cell types, additional chemical fluorophores can be introduced to enhance the fluorescent signal. These supplementary fluorophores are versatile, responding to different excitation wavelengths and emitting light across a wide range of colors in the visible spectrum [12].
The green and red autofluorescence characteristics of enamel, dentin, and dental plaque have been documented for over a century [13]. In dentistry, numerous specialties benefit from autofluorescence applications, ranging from carious lesion detection to oral cancer screening. Early detection, diagnosis, and prediction of lesion activity through this technique have garnered substantial interest. Notably, mature plaque exhibits red autofluorescence when illuminated with 405 nm blue light [14]. This red autofluorescence signature appears in various pathological conditions, including teeth with plaque accumulation, calculus deposits, infected implant surfaces, active caries, and stomatitis [4,15-19]. In contrast, healthy tissues display different spectral characteristics [13]. In oral cancer applications, normal soft tissue exhibits fluorescence, while potentially malignant lesions appear as dark regions [2].
Real-time, hands-free detection of infected tissues can be accomplished using a novel fluorescence-based magnification loupe equipped with a 405 nm LED light source: Reveal® (DesignsForVision Inc, NYC, New York, USA). This system is customized to the operator's specific optical requirements and provides magnification that overcomes limitations in visual, tactile, and radiological diagnosis. It embodies the theragnosis concept (combining diagnostic and therapeutic modalities), enabling comprehensive treatment guidance from initial diagnosis through the differentiation of healthy and diseased tissues, to treatment completion [20].
What is Fluorescence and How Does it Work?
Fluorescence is a physicochemical energy exchange phenomenon where molecules absorb shorter-wavelength photons and re-emit a part of their energy as longer wavelength photons. The surface or object containing the pigment molecule appears to glow due to this energy conversion, rather than through actual light production [10]. Bioluminescence, a related phenomenon, refers to the production of light by living organisms, occurring naturally across various biological kingdoms, including plants, animals, and humans [21-26].
The bioluminescent light emission process involves various enzymes and light-emitting molecules, notably luciferases and luciferins. The spectral range of this emission spans from 400 to 700 nm, corresponding to blue through red light. Importantly, the resulting fluorescence may differ from the object's intrinsic color reflection. While ultraviolet light is commonly associated with fluorescence excitation, blue or blue/green light can also effectively trigger this phenomenon, resulting in green, yellow, or red fluorescence [27].
In the natural settings, two key properties enable fluorescent objects to appear notably brighter than their surroundings. First, the difference between excitation and emission of the fluorophores to their non-fluorescent surroundings may cause the objects to appear brighter in certain spectral regions. This effect is further enhanced by a second property: isotropic emission. Since vertical (downwelling) light intensity typically exceeds horizontal light intensity, fluorescent objects that absorb and re-emit this downwelling light uniformly in all directions appear notably brighter against the horizontal background illumination [28]. To maximize the contrast advantages offered by fluorophores, visual detection systems typically focus on narrow spectral regions [10]. In contrast, the intraocular photoreceptors of the human vision possess a broad spectral sensitivity (400-700 nm), requiring the use of specialized filters for optimal visualization of biofluorescent colors [29].
Bioluminescence in Theragnosis of Oral Cavity
Biofilms represent the predominant bacterial framework in dental infections, consisting of mono- or multi-species bacterial communities embedded within a self-produced extracellular polymeric matrix substance (EPS) [30]. The EPS not only facilitates bacterial adhesion to various surfaces but also provides protection against environmental threats. Within the biofilm structure, bacterial cells frequently develop decreased susceptibility to disinfectants and antibiotics. Additionally, the structure facilitates cell-to-cell communication through quorum sensing. Consequently, biofilms have evolved greater resistance compared to their planktonic counterparts, presenting a significant therapeutic challenge [31-33].
Dental biofilm readily develops on various oral surfaces including teeth, implants, orthodontic brackets, root canals, anatomical gaps, and injured soft tissues. These biofilms constitute the primary etiological factor in numerous dental pathologies including caries, periodontal disease, peri-implantitis, mucositis, endodontic failure, and other oral conditions [32,34-37]. When left untreated, these conditions can progress to cause pain, tooth loss, and potentially affect systemic health [38-44]. The detection of early lesions and monitoring disease progression presents significant challenges, even for experienced clinicians. Traditional diagnostic methods, including radiographs and clinical visual or tactile examination, have limitations in detecting subtle changes, such as distinguishing between early caries progression and remineralization [45]. In this context, theragnostic devices, which combine diagnostic and therapeutic capabilities, offer significant advantages in dental practice. Fluorescence-based adjuncts enable real-time detection of biofilm-related biological parameters through non-invasive, user-friendly methods.
Extensive research has identified various endogenous fluorophores in oral bacteria that emit visible light when exposed to ultraviolet irradiation. For instance, Porphyromonas gingivalis (PG), a Gramnegative anaerobic bacterium implicated in periodontal disease and peri-implantitis [46,47], exhibits characteristic red fluorescence [14,48,49]. Aggregatibacter actinomycetemcomitans (AA), a Gramnegative facultative anaerobic bacterium associated with localized aggressive periodontitis and peri-implantitis, displays yellow to orange spectral emissions [50-52]. Streptococcus mutans (SM), a Gram-positive facultative anaerobic bacterium central to dental caries development, produces green fluorescence [14,48,53]. These distinct spectral signatures result from intrinsic photosensitizers: Protoporphyrin IX and coproporphyrin in PG, and flavin adenine dinucleotide (a bacterial energy metabolism product) in SM and AA [54].
Antimicrobial Light – the Current “Magic Bullet”?
A notable characteristic of biological systems containing photosensitizers is their energy emission from the first excited singlet state as fluorescence. This property enables the use of biofluorescence as an antimicrobial tool, supporting both diagnosis-decision making and treatment of oral conditions. Protoporphyrin IX (PpIX), a photosensitizer that accumulates in certain bacteria due to their lack of Ferochelatase enzyme, stimulates the production of singlet oxygen and Reactive Oxygen Species (ROS) in the presence of a light sensitizer, resulting in cytotoxic effects [55]. 5-Aminolevulinic Acid (5-ALA) is an intrinsic photosensitizing agent (differently from toluidine blue and methylene blue), which is converted into an endogenous substance, Protoporphyrin IX, the penultimate molecule to the formation of heme group. This powerful natural photosensitizing molecule has found extensive application in treating cancerous and pre-cancerous conditions [56-60].
The concept of the "Magic Bullet" originated with Paul Ehrlich over a century ago. He envisioned an antimicrobial agent capable of specifically eliminating pathogenic microorganisms while sparing host tissues. His pioneering work led to the development of the first antibiotic [61]. Ehrlich's work was followed by Alexander Fleming and his serendipitous discovery of penicillin which revolutionized medicine [62]. While antibiotics have undeniably saved millions of lives, they present certain limitations including bacterial resistance, side effects, limited tissue penetration, and potential host damage [62].
Antimicrobial light represents an innovative non-antibiotic approach. Its mechanism relies on photoexcitation of endogenous porphyrins (chromophores) in pathogenic microbes, generating intracellular ROS that induce lipid peroxidation and damage to cellular membranes, proteins, and DNA, ultimately leading to microbial death [63]. This multi-target mechanism shows promise in countering antibiotic resistance. Importantly, antimicrobial light demonstrates significantly less toxicity to host cells compared to UVC irradiation [63]. Safety studies consistently show that antimicrobial light exhibits minimal toxicity to mammalian cells while maintaining effectiveness against pathogens [64-66]. Even at high radiant exposures in vivo (>700 J/cm² combined), the technique does not induce mammalian cell apoptosis [64]. Wavelength selection plays a crucial role in achieving optimal results, with different bacteria responding to various wavelengths [64]. Antimicrobial blue light (aBL; 400-470 nm; violet to pure blue colors) demonstrates significant pathogenic cell killing effects, with 405 nm showing the most potent microbicidal properties [66-72]. While longer wavelengths (532-650 nm; green to red colors) show lower bactericidal activity [68], they offer beneficial host effects, including tissue regeneration and pain control, making them valuable as adjunct therapies [64]. Current literature clearly demonstrates that applied bioluminescence offers potent antimicrobial properties across a broad spectrum of microorganisms while maintaining compatibility with traditional antimicrobial approaches [61-64,66,73,74].
Equipment Presentation
REVEAL™ (Designs for Vision, New York, USA), first proposed in 2017 and launched in January 2020 at the Yankee Dental Meeting in Boston, is an advanced diagnostic system combining filtered telescopic lenses with dual daylight/fluorescence 405 nm light technology. The system's transparent, double-filtering design enables handsfree, real-time fluorescence visualization through a sophisticated optical configuration. This includes a restrictive high-pass 430 nm transparent filter at the distal lens opening and an additional proximal filter that attenuates residual magnified blue light.
The REVEAL™ system's versatility allows integration with various telescopic lenses, including panoramic, expanded-field, and refractive infinity view telescopes. The panoramic and expanded-field telescopes mount onto a Ziena glossy black plastic frame, featuring optional anti-splatter black eyecups for peripheral sealing during procedures. The 4.5x Panoramic loupes with Dual Headlight maintain practical usability at 5.9oz/167.3g, while achieving approximately 91% visible light transmission.
The system features a vertically stacked dual headlight attached directly to the loupe frame, delivered in a protective zippered fabric case with conventional foam securing. The battery-powered headlight offers two operating modes:
Single press: Activates conventional white light from the top beam, providing 32,100 lux illumination suitable for all dental procedures; Double press: Engages the 405 nm wavelength bottom beam, activating the fluorescence function
The 405 nm narrow spectrum light stimulates bacterial byproducts associated with caries, calculus, and peri-implantitis, inducing endogenous fluorescence. The filtered LED light system effectively contains energetic light within the housing while transmitting the desired wavelength. The system's minimal heat generation enables extended operational procedures under fluorescence guidance without requiring ambient light reduction.
The complete REVEAL™ Fluorescence Guided Dentistry system comprises:
Reveal Headlight
White headlight: 32,100 lux illumination for standard procedures; Blue excitation headlight: 405 nm narrow spectrum excitation light for bacterial byproduct fluorescence.
Reveal Eyewear
Excitation wavelength filtering for clear fluorescence visualization; 430 nm high-pass filter with 91% visible light transmission; Integrated professional eyeglass prescription.
Magnification
Customized magnification for optimal hands-free operation; Precisely positioned telescopes with through-lens drilling; Adjusted to professional's interpupillary distance; Integrated eyeglass prescription.
Battery Pack
High-capacity power source; User-friendly activation; Compact and lightweight design (Figure 1).
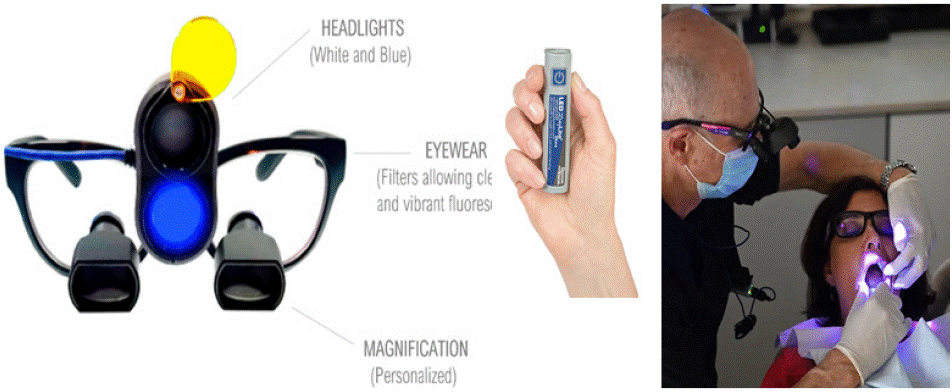
Figure 1: REVEAL loupes and battery, and clinical view when wearing the loupes.
Real time analysis: REVEAL™ Fluorescence Guided Dentistry System allows you to:
1. Visualize the byproducts of bacteria associated with caries, calculus, peri-implantitis without the use of any pharmaceutical, chemical or dye;
2. Control and confirm the complete removal of mature biofilm during prophylaxis;
3. Identify and differentiate between active and arrested caries lesions, preserving more tooth structure;
4. Diagnose and manage cariology, oral-hygiene, eriodontology, implantology, restorative dentistry and oral medicine.
Case Series Using Reveal™
Cases 1 and 2 - Oral Hygiene and Patient Motivation (Figure 2)
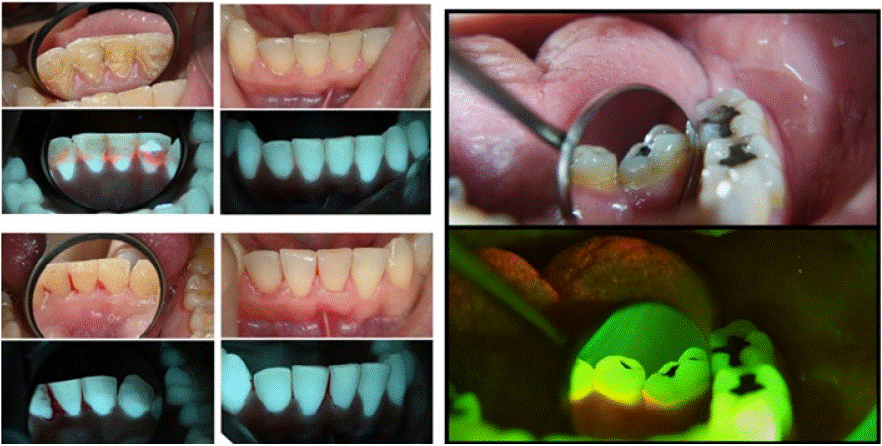
Figure 2: Plaque detection and oral hygiene; Lower front teeth lingually (left) and lower molar and premolars (right) with regular light and using ReVeal.
Case 1 Description
A female patient, 30 years old. History of orthodontic treatment. No systemic conditions or relevant medical history. The patient's chief complaint was a persistent sensation of roughness on the lingual surfaces of her mandibular anterior teeth.
Findings: White-light: calculus deposits were observed on the gingival third of the teeth, associated with gingival inflammation. Additionally, remnants of composite restorations were observed, presumably securing a past orthodontic lingual splint. Minor plaque deposits were observed on the facial aspect of the anterior incisors, especially on the lower third.
Fluorescence-light: bright red autofluorescent calculus deposits were identified, as well as numerous restorative materials with varied degrees of fluorescence. The inflamed gingiva showed decreased tissue autofluorescence. Red autofluorescence was observed from the plaque deposits on the facial aspect if the anterior incisors.
Post operative description: The removal of the red-fluorescence calculus deposits and fluorescent restorative materials were confirmed.
Case 2 Description
A female patient, 63 years old, with no systemic conditions, smokes 3 cigarettes per day. Her chief complaint was the need for habitual mouth cleaning procedure.
Findings: White-light displayed prominent pigmentation on the cervical and gingival thirds of the teeth. Minor plaque deposit was identified on the disto-lingual surface of 36. The plaque appeared transparent.
Fluorescence-light: The mature plaque which appeared transparent under white-light, was easily noticed under fluorescence conditions. Additionally, patched of additional plaque were identified on the adjacent teeth.
Educational perspective: Localizing biofilm with ReVeal is possible regardless of the location, and the easy view enhances theragnosis. The easy identification of plaque and calculus allows dental students and clinicians to identify the dental biofilm, and target the cleaning procedure. Magnification with loupes enhances visibility and better addresses the exact areas to reach. Patients are able to observe more clearly where the biofilm is present and where they should place effort to minimize its presence.
Cases 3 and 4 - Oral hygiene and patient motivation (Figure 3)
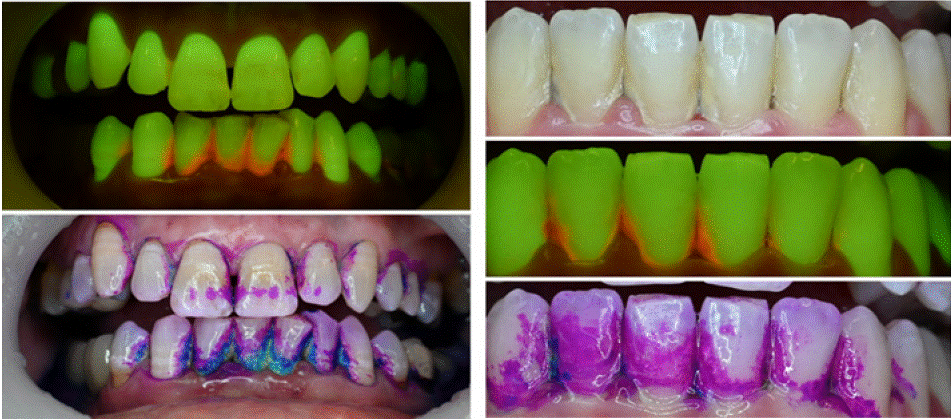
Figure 3: Cases 3 (left) and 4 (right), in which bioluminescence shows areas with biofilm, whereas regular stains are coloring not specific areas.
Case 3 Description
A male patient, 61 years old, with no systemic conditions or relevant medical history. His chief complaint was bleeding in the lower anterior region when brushing his teeth.
Findings: White-light showed substantial yellow calculus deposits and plaque associated with gingival inflammation in the lower anterior region. After staining with TriPlaque ID gel™, abundant areas of fresh, mature and acidogenic plaque were observed. The hard calculus deposits showed only partial staining, appearing as a light pale/white shade. Soft tissues were also stained by the dye.
Fluorescence- light: abundant red fluorescence was observed from the calculus deposits on the surface of the lower anterior teeth. Gingival inflammation was clearly observed in these regions, characterized by diffused dark-red regions of decreased natural tissue autofluorescence
Case 4 Description
A female patient, 27 years old, with general good health, without systemic conditions. Her chief complaint was bleeding in the lower anterior region.
Findings: White-light showed overall good oral hygiene, apart from calculus deposits on the surface of the lower anterior teeth. After staining with TriPlaque ID gel™, fresh plaque was predominantly observed with scarce points of mature plaque. The calculus was only partially stained, and was mainly covered with fresh plaque.
Fluorescence- light: red fluorescence was observed from the calculus deposits and mature plaque even though it was visually indiscernible with the multiple-tone plaque disclosing dye
Educational perspective: Just as cases 1 and 2, understanding where the biofilm is present allows better hygiene procedures from dental students, clinicians and patients. Current stains are not specific and may leave blurred areas which are not actually dental biofilm. It is important to differentiate the biofilm from stained area, and fluorescence approaches provide that.
Case 5 – Caries Diagnosis and Treatment (Figure 4)
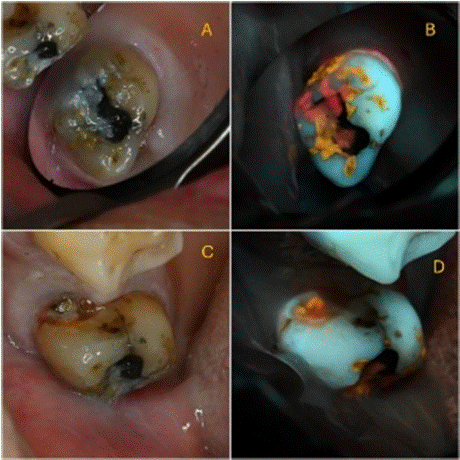
Figure 4: Caries detection (A) white-light occlusal view; (B) 405 nm
fluorescence occlusal view; (C) white-light mesial view; (D) 405 nm
fluorescence mesial view.
Case 5 Description
A female patient, 70 years old, with good general health, without relevant medical history. Her chief complaint was sensitivity to cold from the upper right second molar (17).
Findings: White-light: Abundant plaque was observed on the occlusal surface of 17. A mesial cavity with visible dentin was observed, corresponding to ICDAS code 5.
Fluorescence- light: abundant red and orange autofluorescence plaque was observed on the occlusal surface of the tooth, as well as from within the observed cavity. Additionally, red autofluorescent calculus was observed on the gingival third into the gingival sulcus.
Educational perspective: Porphyrin from biofilm will be spotted regardless if it is plaque, caries or calculus. Following oral hygiene, only caries will remain, demanding proper intervention.
Case 6 – Caries Diagnosis and Treatment (Figure 5)
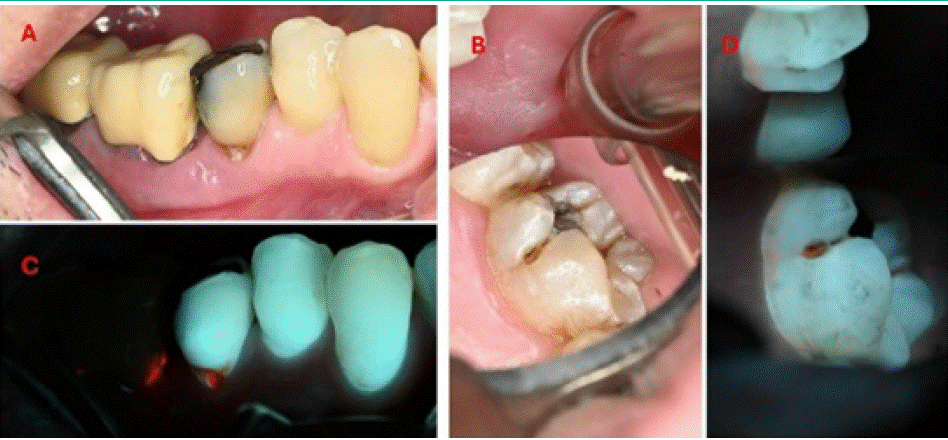
Figure 5: Caries seen under white-light (A and B) and 405 nm fluorescence (C and D).
Case Description A and B
A male patient, 75 years old, with good general health, without relevant medical history. The patient attended an oral hygiene appointment.
Findings: White-light: A root-caries lesion was observed on tooth 35, with distinct cavity reaching dentin (ICDAS code 5). Subgingival plaque was observed on tooth 36.
Fluorescence- light: Intense red autofluorescence was observed from within the root-caries lesion. We could observe the lack of natural fluorescence from the crowns covering teeth 36-37. Easily notable red fluorescence was observed from the subgingival sulcus, which stem from an accumulation of plaque and calculus. Note that white spot lesions on teeth display a brownish color.
Case Description C and D
A male patient, 36 years old, with good general health, smokes 2 cigarettes a day. The patient attended an oral examination.
Findings: White-light: A palatal lesion was observed on 26. Minor plaque deposits were observed on the gingival region of the tooth. The cavity had localized enamel breakdown, without visible dentin, corresponding ICDAS code 3.
Fluorescence- light: Orange fluorescence was observed from within the cavity. Additionally, a thin band of red auto fluorescent plaque was observed on the gingival region of the tooth.
Educational perspective: Caries are better viewed under fluorescence. Areas that would maybe not identified in a regular visit are seen exactly where it is located, due to porphyrin identification.
White spot lesions on teeth displays a brownish color. These should be cleaned and a remineralization approach should be considered.
Cases 7 And 8 – Caries Diagnosis and Treatment (Figure 6)
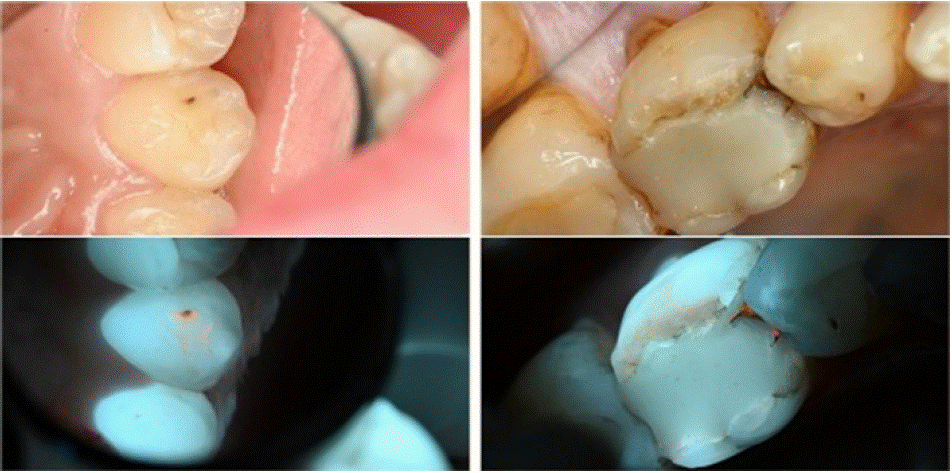
Figure 6: White-light views (above) and 405 nm fluorescence views (below).
Case 7 Description
A female patient, 20 years old, with good general health. The patient attended an oral examination.
Findings: White-light: Examination revealed an enamel lesion on the palatal surface of tooth 23 (left maxillary canine), accompanied by distinct discoloration.
Fluorescence- light: Orange fluorescence was observed on the surface of the lesion, indicating its activity. Additional orange fluorescence was observed in the pits and fissures, which were invisible under white-light conditions.
Case 8 Description
A male patient, 75 years old., with good general health. His chief complaint was sensitivity to cold drinks from the first lower left molar.
Findings: White-light: A crack at the mesial margin of the restoration was observed, additionally the edges of the restoration not adapted to the current anatomy of the tooth surface.
Fluorescence- light: Orange red autofluorescence was observed under the existing restoration on the mesial surface. Additionally, orangish autofluorescence was observed on the surface of the buccal facing cusps.
Educational perspective: some difficult to find caries are present, and under regular white light they are not always identifiable. Under 405 nm light porphyrin glows as orange/red areas and help identifying where to remove/treat.
Case 9 – Caries Diagnosis and Treatment (Figure 7)
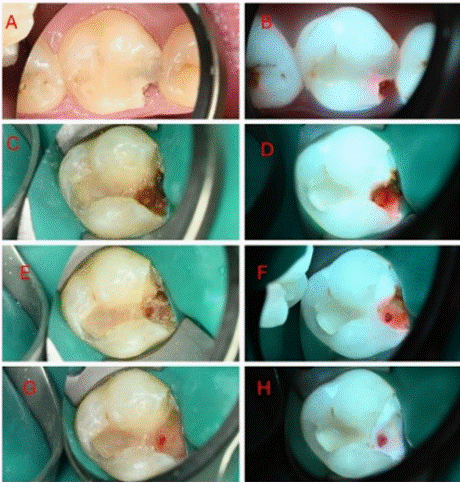
Figure 7: White-light initial occlusal view (B) 405 nm fluorescence, initial
occlusal view (C) white-light cavity opening (D) 405 nm fluorescence, cavity
opening (E) white-light, first excavation (F) 405nm, first excavation (G)
white-light, second excavation (H) 405 nm, second excavation.
Case Description
A female patient, 19 years old, with good general health. Her chief complaint was pain to cold drinks from the upper region. Periapical radiograph showed possible involvement of the pulp horn.
Findings: White-light: An expansive cavitated lesion was observed on the mesial aspect of tooth 16. (A). Following the cavity opening, the extent on the lesion was observed with the presence of a small avascular tissue area, indicating necrosis. (C) A gradual excavation (E, G) was carried out to attain the removal of the caries tissue to sound hard tissue to allow for a quality adhesive restoration prior to carrying out the endodontic treatment.
Fluorescence- light: Red autofluorescence was observed under the existing restoration resulting with a red hale emanating from within the tooth (B). Following the cavity opening, a substantial infiltration of red bacterial autofluorescence was observed, reaching the pulp (D) avascular area. A gradual excavation was carried out to sound hard tissue, free of bacterial red autofluorescence. (F, H).
Educational perspective: Caries removal can be performed only where porphyrin is displayed as red/orange light, allowing minimally invasive procedure. Clinician is able to take decision based on a clear standard. Theragnosis (diagnosis + treatment) is performed preserving safe and sound tissue structures.
Cases 10 And 11– Periodontal Disease (Figure 8)
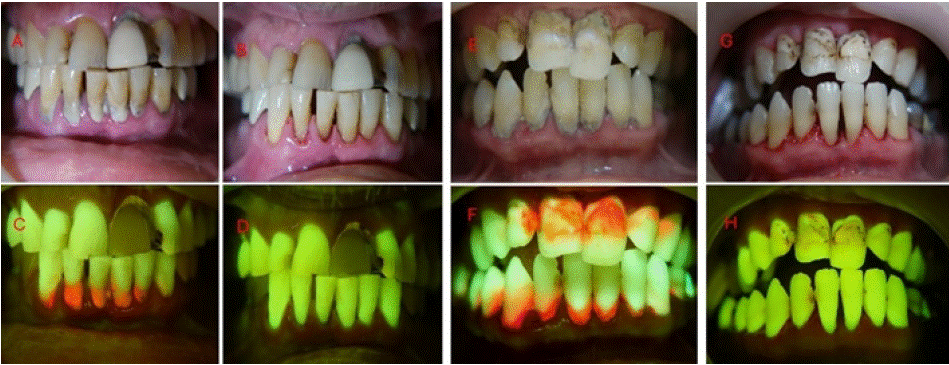
Figure 8: (A) White-light frontal view before scaling (B) 405 nm fluorescence frontal view before scaling (C) white-light frontal view after scaling (D) 405 nm
fluorescence view after guided scaling (E) white-light frontal view before scaling (F) 405 nm fluorescence frontal view before scaling (G) white-light frontal view
after scaling (H) 405 nm fluorescence view after guided scaling.
Case 10 Description
A male patient, 61 years old, smokes +-7 cigarettes a day. The patient's primary concern was persistent oral malodor.
Findings: White-light: poor oral hygiene habits of the patient were observed, with substantial calculus deposits associated with gingival inflammation in the lower anterior region.
Fluorescence- light: intense red and orange bacterial autofluorescence were observed from the calculus deposits on the surface of the anterior teeth. Intense gingival inflammation was clearly observed with these associated areas, characterized by diffused darkred regions of decreased natural tissue autofluorescence. Additionally, a metal-ceramic crown with a faint autofluorescence was observed on the left upper central incisor, surrounded by a composite restoration with autofluorescence characteristics and an inflammatory band with decreased tissue autofluorescence. An additional cavitated lesion was spotted on the left upper lateral incisor which would require treatment in a separate session.
Post operative: Using Reveal™, we could confirm in real-time, and in a direct manner, the removal of calculus deposits and objectively evaluate the fulfillment of the treatment goal.
Case 11 Description
A male patient, 52 years old, with good general health, without systemic conditions. He presented to an oral hygiene treatment at the request of his wife.
Findings: White-light: The patient was not bothered by his oral hygiene nor did he engage in regular self-hygiene oral habits. substantial plaque and calculus covering the majority of the patient’s teeth as well as the presence of extrinsic stains and lesions suspected as caries. Generalized gingival inflammation was observed as well.
Fluorescence- light: intense red bacterial autofluorescence was observed from calculus deposits located on the gingival margins of the teeth. Additionally, substantial diffused areas of mature plaque covered the smooth surfaces of the teeth. Intense general gingival inflammation was also observed in the patient, characterized by diffused dark-red regions of decreased natural tissue autofluorescence.
Post operative: Following the use of Reveal™, we could objectively confirm the removal of the plaque and calculus deposits as well as locate active caries lesions which would require additional care; an infiltrating right upper central incisor restoration and cavitated lesions on the mesial aspect of the left lower lateral incisor and canine.
Educational perspective: Plaque and calculus removal can be performed where porphyrin is displayed as red/orange light, allowing a procedure that allows total and safe removal of the biofilm. Clinician is able to repeat scaling and cleaning until it is good enough. Theragnosis (diagnosis + treatment) is performed to allow better quality cleaning.
Case 12 – Periimplantitis (Figure 9)
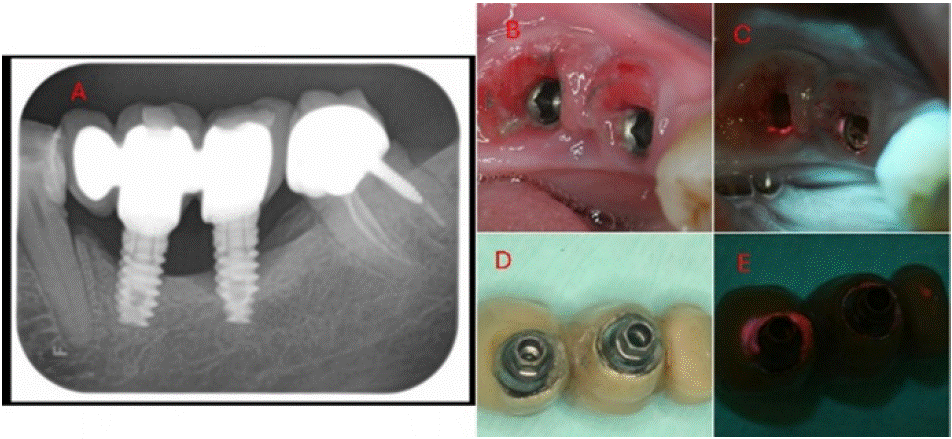
Figure 9: (A) X-ray (B) white-light view of the exposed implants under the prosthetic (C) 405 nm fluorescence view of the exposed implants under the prosthetic
(D) white-light inferior view of the implant supported prosthetic (E) 405 nm fluorescence view of the implant supported prosthetic.
Case Description
A female patient, 56 years old, with good general health, without relevant medical history. Her chief complaint was pain and bleeding from the gums in the area of the implant-supported prosthetic. The X-ray showed localised horizontal bone loss at the location of the implants, with a vertical defect attributed to advanced peri-implantitis on the implant located at 37.
Findings: White-light: Substantial inflammation was observed in the gingiva adjacent to the implants. Additionally, dental plaque accumulated around the implants. (B). The implant supported prosthesis was cemented to the abutments. A wide gap was observed at the abutment-prosthesis interface, resulting with plaque and calculus accumulation. (D). Fluorescence- light: Red bacterial autofluorescence was observed surrounding the implants and penetrating the gingival sulcus. Abundant red autofluorescence was observed from the accumulation of plaque and calculus in the gap of the abutment-prosthesis interface.
Educational perspective: Clinician is able to identify clearly where the problem stands, helping provision of adequate decision making towards the case. Note that red autofluorescence denotes a mature biofilm, determining a long-standing problem that can be sorted with adequate treatment.
Cases 13 and 14 – Periimplantitis (Figure 10)
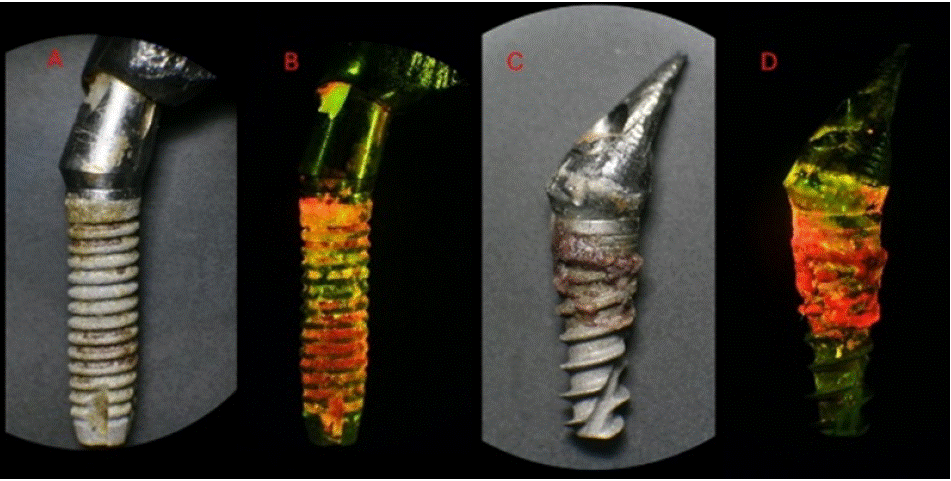
Figure 10: (A) White-light photography of a freshly pulled failed dental implant; (B) 405 nm fluorescence view of the failed implant; (C) White-light photography
of another freshly pulled failed dental implant (D) 405 nm fluorescence view of the failed implant. Note that the extent of infection is clearly seen under 405 nm
view.
Case 13 Description
A female patient, 82 years old, with general good health. The patient had a pacemaker. Her chief complaint was sensitivity in the posterior region of lower right side with crown mobility. Clinical diagnosis was of a failed dental implant which was pulled out of the gingival fibrous capsule with tweezers.
Image description: White-light: calculus contaminants were observed non-uniformly on the surface of the implant, predominantly on the upper third.
Fluorescence- light: Abundant bacterial autofluorescence was observed covering the entire surface of the implant, including regions which appeared non-contaminated under white-light. The autofluorescence had multiple shades of red, orange, and yellow, as well localized spots of green autofluorescence.
Case 14 Description
A female patient, 71 years old, with general good health. Her chief complaint: crown mobility in an implanted supported restoration located at 24. Clinical diagnosis was failed dental implant which was pulled out of the gingival fibrous capsule with tweezers.
Image description: White-light: calculus contaminants were on the surface of the upper half of the implant.
Fluorescence- light: Intense red autofluorescence was observed from the contaminated surfaces. The autofluorescence had shades of red, orange and yellow, which was also present at the implantabutment interface and the dental abutment.
Educational perspective: Clinician can visualize the infection and also show the patient where the problem is. Red autofluorescence denotes a mature biofilm, determining a long-standing situation that led to failure in the two cases above.
Case 15 – Oral Medicine (Figure 11)
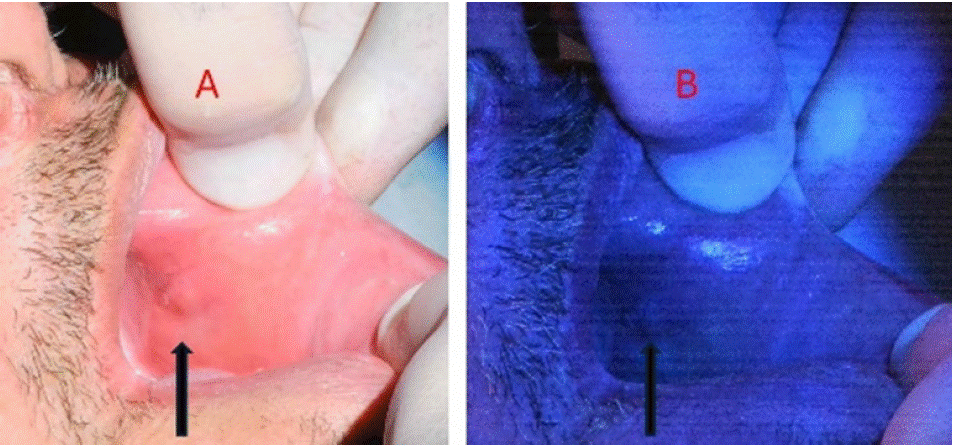
Figure 11: (A) Patient with a white patch and rough surface suggestive of oral leukoplakia; (B) 405 nm view displayed an area with lack of fluorescence in oral tissue.
Case 15 Description
Male patient, 52 years old, heavy smoker and alcoholic for more than 30 years, was seen for a regular dental visit. Clinical examination of the oral mucosa revealed a white, rough-textured lesion. Clinical initial diagnosis was of leukoplakia. Biopsy was performed and histopathological diagnosis was of oral squamous cell carcinoma.
Image description: White-light: a slightly white patch could be seen at the left cheek.
Fluorescence- light: loss of fluorescence slightly above the white patch led to the decision to perform a biopsy in that area. The diagnosis was of oral squamous cell carcinoma.
Educational perspective: oral tissues have natural fluorescence. Proteins like collagen, elastin, keratin and tryptophan are some of the fluorophores in oral mucosa. Disruption of oral tissues lead to loss of natural fluorescence, and are indicative of a potentially malignant disorder. The use of 405 nm fluorescence light helps indicating the best areas to perform biopsy.
Case 16 – Oral Medicine (Figure 12)
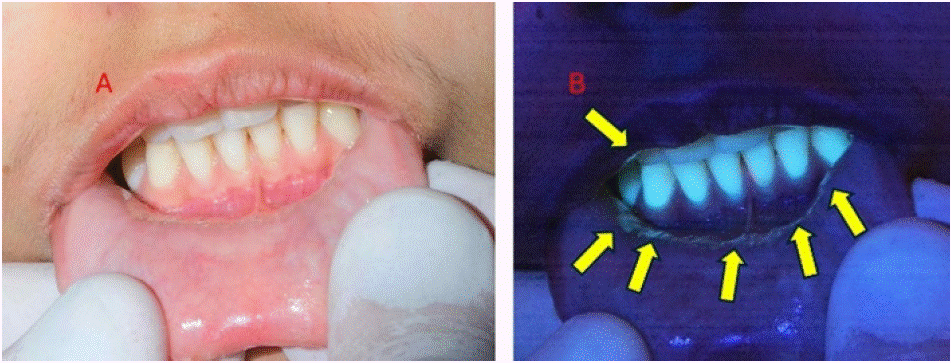
Figure 12: (A) Patient with grade 3 oral mucositis at the oral vestibule and lower lip; (B) 405 nm view displayed infected areas (arrows).
Case 16 Description
Female patient, 19 years old, subjected to treatment with methotrexate for osteosarcoma. Main complaint was sensitivity and pain to eat at the area of oral vestibule and lower lip. When examining oral mucosa, a grade 3 oral mucositis could be seen.
Image description: White-light: red ulcer could be seen in the area near lower incisors at the vestibule and lower lip.
Fluorescence- light: Under fluorescence examination, the ulcer was circumscribed by a distinctive blue-green halo, indicating the presence of infected tissue.
Educational perspective: despite mucositis was visible with regular white light, the contour with infected tissue was strongly shown under fluorescence 405 nm light. It allows the local medication to be applied in the correct places, facilitating procedures to reduce pain, inflammation and infection.
Case 17 – Root Fracture (Figure 13)
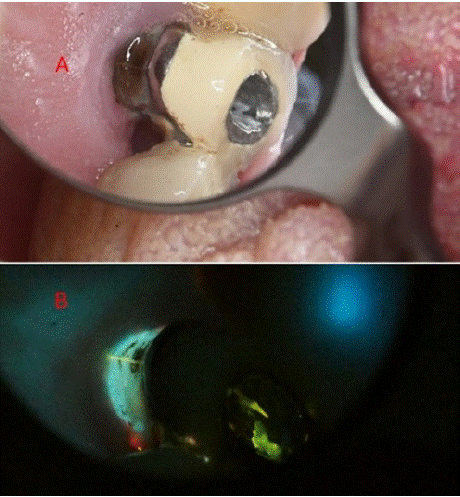
Figure 13: (A) Tooth with crown and under root canal treatment (B) Under
405 nm light and with the aid of Ledermix, a root fracture line became
evident.
Case 17 Description
Male, 68 years old, attended due to intense pain on mastication and touch.
Image description: White-light: an endodontically treated tooth with notable darkening and an old crown was observed. An access cavity was performed through the crown, until the GP to observe the pulpal chamber. Ledermix was applied at the base of the chamber and a temporary filling was placed.
Fluorescence- light: a day afterwards, clear infiltration of the fluorescent paste Ledermix infiltrated the suspected fracture line advancing subgingivally. Clear sign of inflammatory band at the gingiva surrounding the tooth was evident.
Educational perspective: the aid of fluorescent dyes aid to the view of conditions such as root fractures. In this case, Ledermix contains tetracycline, which is fluorescent. Many other fluorophores can be used to aid visualization of root fractures.
Discussion
Fluorescence applications in dentistry have garnered increasing attention within the scientific community, particularly for their potential as educational tools in both undergraduate and graduate dental education. The integration of fluorescence technology into hands-free optical devices such as the "Reveal" represents a methodological advancement in how clinicians can approach diagnosis and treatment, eliminating the need for handheld instruments during procedures. The implementation of fluorescencebased techniques in clinical and educational environments demonstrates favorable learning dynamics, with practitioners rapidly adapting to the diagnostic modality. Understanding the underlying principles of fluorescent emissions enhances the application scope, allowing educators to demonstrate oral pathologies in real-time. This capacity for immediate visualization of typically obscure conditions provides valuable learning opportunities in dental education.
Multiple applications across various dental specialities demonstrate the versatility of fluorescence-based diagnosis and monitoring. The ability to visualize bacterial colonization, tissue changes, and pathological processes in real-time supports evidencebased clinical decision-making. A key consideration for clinical implementation is the need to optimize ambient lighting conditions during fluorescence visualization at the 405 nm wavelength, as excessive environmental light may reduce the technique's diagnostic efficacy. This consideration should be factored into clinical protocols and educational methodology.
Conflict of Interests
Liviu Steier owns IP rights for ReVealTM.. All the other authors deny any conflict of interest.
Informed Consent
All patients signed the informed consent form, allowing publication of the images.
References
- White JM, Eakle WS. Rationale and Treatment Approach In Minimally Invasive Dentistry. The Journal of the American Dental Association. 2000; 131: 13S-19S.
- Tatehara S, Satomura K. Non-Invasive Diagnostic System Based on Light for Detecting Early-Stage Oral Cancer and High-Risk Precancerous Lesions— Potential for Dentistry. Cancers. 2020; 12: 3185.
- Joseph B, Gopalakrishnan S, Alamoudi RA, Pachathundikandi SK, Alotaibi RN, Anil S. Detection of invisible dental biofilm using light-induced autofluorescence in adult patients-A systematic review. Photodiagnosis Photodyn Ther. 2022; 39: 102916.
- Buchalla W, Lennon AM, Attin T. Fluorescence spectroscopy of dental calculus. J Periodontal Res. 2004; 39: 327-332.
- Fee PA, Macey R, Walsh T, Clarkson JE, Ricketts D. Tests to detect and inform the diagnosis of root caries. Cochrane Database Syst Rev. 2020; 12: Cd013806.
- Macey R, Walsh T, Riley P, Glenny AM, Worthington HV, Fee PA, et al. Fluorescence devices for the detection of dental caries. Cochrane Database Syst Rev. 2020; 12: Cd013811.
- Ghodasra R, Brizuela M. Dental Caries Diagnostic Testing. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC. 2023.
- Heinrich-Weltzien R, Kühnisch J Fau - Ifland S, Ifland S Fau - Tranaeus S, Tranaeus S Fau - Angmar-Månsson B, Angmar-Månsson B Fau - Stösser L, Stösser L. Detection of initial caries lesions on smooth surfaces by quantitative light-induced fluorescence and visual examination: an in vivo comparison. 2005; 113: 494-498.
- Oh SH, Choi JY, Kim SH. Evaluation of dental caries detection with quantitative light-induced fluorescence in comparison to different field of view devices. Sci Rep. 2022; 12: 6139.
- Marshall JA-O, Johnsen SA-O. Fluorescence as a means of colour signal enhancement. LID - LID - 20160335. 2017: 372.
- Uttamlal M, Sheila Holmes-Smith A. The excitation wavelength dependent fluorescence of porphyrins. Chemical Physics Letters. 2008; 454: 223-228.
- Walsh LJ, Shakibaie F. Ultraviolet-induced fluorescence: shedding new light on dental biofilms and dental caries. Australasian Dental Practice. 2007: 56- 60.
- König K, Flemming G, Hibst R. Laser-induced autofluorescence spectroscopy of dental caries. Cell Mol Biol (Noisy-le-grand). 1998; 44: 1293-1300.
- Coulthwaite L, Pretty IA, Smith PW, Higham SM, Verran J. The microbiological origin of fluorescence observed in plaque on dentures during QLF analysis. Caries Res. 2006; 40: 112-116.
- Thomas RZ, van der Mei HC, van der Veen MH, de Soet JJ, Huysmans MC. Bacterial composition and red fluorescence of plaque in relation to primary and secondary caries next to composite: an in situ study. Oral Microbiol Immunol. 2008; 23: 7-13.
- van der Veen MH, Thomas RZ, Huysmans MC, de Soet JJ. Red autofluorescence of dental plaque bacteria. Caries Res. 2006; 40: 542-545.
- Shigetani Y, Takenaka S, Okamoto A, Abu-Bakr N, Iwaku M, Okiji T. Impact of Streptococcus mutans on the generation of fluorescence from artificially induced enamel and dentin carious lesions in vitro. Odontology. 2008; 96 :21-25.
- Ku HM, Jun MK, Kim JH, Kwon HK, Kim BI. Explaining the Red Fluorescence Evident on the Surface of Failed Dental Implants: Case Reports. Implant Dent. 2016; 25: 445-449.
- Volgenant CM, Hoogenkamp MA, Krom BP, Janus MM, Ten Cate JM, de Soet JJ, et al. Red and Green Fluorescence from Oral Biofilms. PLoS One. 2016; 11: e0168428.
- Steier L, Figueiredo JAP, Blatz MB. Fluorescence-Enhanced Theragnosis: A Novel Approach to Visualize, Detect, and Remove Caries. 2021; 42: 462-465.
- Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000; 408: 850-853.
- Weagle G, Paterson PE, Kennedy J, Pottier R. The nature of the chromophore responsible for naturally occurring fluorescence in mouse skin. J Photochem Photobiol B. 1988; 2: 313-320.
- Iriel A, Lagorio MG. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften. 2010; 97: 915-924.
- Mazel CH, Cronin TW, Caldwell RL, Marshall NJ. Fluorescent enhancement of signaling in a mantis shrimp. Science. 2004; 303: 51.
- Stachel SJ, Stockwell SA, Van Vranken DL. The fluorescence of scorpions and cataractogenesis. Chem Biol. 1999; 6: 531-539.
- Ozawa T, Yoshimura H, Kim SB. Advances in fluorescence and bioluminescence imaging. Anal Chem. 2013; 85: 590-609.
- Johnsen S. The optics of life: a biologist’s guide to light in nature.: Princeton, NJ: Princeton University Press.; 2012: 65.
- Vukusic P, Hooper I. Directionally controlled fluorescence emission in butterflies. Science. 2005; 310: 1151.
- Konatham S, Martín-Torres J, Zorzano MP. The Impact of the Spectral Radiation Environment on the Maximum Absorption Wavelengths of Human Vision and Other Species. Life (Basel). 2021; 11.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010; 8: 623-633.
- Majumdar S, Pal S. Cross- species communication in bacterial world. J Cell Commun Signal. 2017; 11: 187-190.
- Abusrewil S, Alshanta OA, Albashaireh K, Alqahtani S, Nile CJ, Scott JA, et al. Detection, treatment and prevention of endodontic biofilm infections: what’s new in 2020? Critical Reviews in Microbiology. 2020; 46: 194-212.
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001; 9: 34-39.
- Dhaliwal JS, Abd Rahman NA, Ming LC, Dhaliwal SKS, Knights J, Albuquerque Junior RF. Microbial Biofilm Decontamination on Dental Implant Surfaces: A Mini Review. Front Cell Infect Microbiol. 2021; 11: 736186.
- Müller LK, Jungbauer G, Jungbauer R, Wolf M, Deschner J. Biofilm and Orthodontic Therapy. Monogr Oral Sci. 2021; 29: 201-213.
- Prada I, Micó-Muñoz P, Giner-Lluesma T, Micó-Martínez P, Collado- Castellano N, Manzano-Saiz A. Influence of microbiology on endodontic failure. Literature review. Med Oral Patol Oral Cir Bucal. 2019; 24: e364-e372.
- Siqueira JF, Jr., Rôças IN. Present status and future directions: Microbiology of endodontic infections. Int Endod J. 2022; 55: 512-530.
- Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019; 19: 176.
- Cotti E, Mercuro G. Apical periodontitis and cardiovascular diseases: previous findings and ongoing research. Int Endod J. 2015; 48: 926-932.
- Hansen PR, Holmstrup P. Cardiovascular Diseases and Periodontitis. Adv Exp Med Biol. 2022; 1373: 261-280.
- Orlandi M, Graziani F, D’Aiuto F. Periodontal therapy and cardiovascular risk. Periodontol. 2020; 83: 107-124.
- Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020; 83: 90-106.
- Coelho C, Mead M. Sepsis: the applicability to dental care professionals. Br Dent J. 2018; 225: 1078-1081.
- Dave M, Barry S, Coulthard P, Daniels R, Greenwood M, Seoudi N, et al. An evaluation of sepsis in dentistry. Br Dent J. 2021; 230: 351-357.
- Meyer-Lueckel H, Paris S, Ekstrand K. Caries Management – Science and Clinical Practice. STOMATOLOGY EDU JOURNAL. 2015; 2: 170.
- Hiltch G, Steier L, de Figueiredo JAP. Enhanced Clinical Decision-Making and Delivery of Minimally Invasive Care Using the ICCMS4D Integrated with Hands-Free Fluorescence-Based Loupes and a Chemomechanical Caries Removal Agent. Eur J Dent. 2023; 17.
- Hwang G, Blatz MB, Wolff MS, Steier L. Diagnosis of Biofilm-Associated Peri-Implant Disease Using a Fluorescence-Based Approach. Dent J (Basel). 2021; 9.
- Yan YJ, Wang BW, Yang CM, Wu CY, Ou-Yang M. Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans. Dent J (Basel). 2021; 9.
- Volgenant CM, van der Veen MH, de Soet JJ, ten Cate JM. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur J Oral Sci. 2013; 121: 156-161.
- Bjurshammar NJ, Annsofi Buhlin, Kåre, Tranæus SÖ, Conny. On the red fluorescence emission of Aggregatibacter actinomycetemcomitans. 2012: 299-30.
- How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016; 7: 53.
- Fine DH, Patil AG, Velusamy SK. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front Immunol. 2019; 10: 728.
- Lee ES, Yim HK, Lee HS, Choi JH, Kwon HK, Kim BI. Plaque autofluorescence as potential diagnostic targets for oral malodor. J Biomed Opt. 2016; 21: 85005.
- Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005; 11: 227-256.
- Roguin LP, Chiarante N, García Vior MC, Marino J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int J Biochem Cell Biol. 2019; 114: 105575.
- Bohm GC, Gándara L, Di Venosa G, Mamone L, Buzzola F, Casas A. Photodynamic inactivation mediated by 5-aminolevulinic acid of bacteria in planktonic and biofilm forms. Biochem Pharmacol. 2020; 177: 114016.
- Wachowska M, Muchowicz A, Firczuk M, Gabrysiak M, Winiarska M, Wanczyk M, et al. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. 2011; 16: 1420-3049.
- Foged C, Haedersdal M, Bik L, Dierickx C, Phillipsen PA, Togsverd-Bo K. Thermo-Mechanical Fractional Injury Enhances Skin Surface- and Epidermis- Protoporphyrin IX Fluorescence: Comparison of 5-Aminolevulinic Acid in Cream and Gel Vehicles. Lasers Surg Med. 2021; 53: 622-629.
- Ding A, Li C, Zhang J. Topical 5-aminolevulinic acid photodynamic therapy in the treatment of verruca plana: Report of 6 cases. Photodiagnosis Photodyn Ther. 2021; 35: 102438.
- Radunovic M, Petrini M, Vlajic T, Iezzi G, Di Lodovico S, Piattelli A, et al. Effects of a novel gel containing 5-aminolevulinic acid and red LED against bacteria involved in peri-implantitis and other oral infections. J Photochem Photobiol B. 2020; 205: 111826.
- Schwartz RS. Paul Ehrlich’s magic bullets. N Engl J Med. 2004; 350: 1079- 1080.
- Sir Alexander Fleming-Discoverer of Penicillin. Cal West Med. 1945; 63: 153.
- Wang Y, Murray CK, Hamblin MR, Hooper DC, Dai T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resistance Updates. 2017; 33-35: 1-22.
- Leanse LG, dos Anjos C, Mushtaq S, Dai T. Antimicrobial blue light: A ‘Magic Bullet’ for the 21st century and beyond? Advanced Drug Delivery Reviews. 2022; 180.
- Ferrer-Espada R, Liu X, Goh XS, Dai T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front Microbiol. 2019; 10: 721.
- Bumah VV, Masson-Meyers DS, Awosika O, Zacharias S, Enwemeka CS. The viability of human cells irradiated with 470-nm light at various radiant energies in vitro. Lasers Med Sci. 2021; 36: 1661-1670.
- Tsutsumi-Arai C, Arai Y, Terada-Ito C, Imamura T, Tatehara S, Ide S, et al. Microbicidal effect of 405-nm blue LED light on Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin. Lasers Med Sci. 2022; 37: 857-866.
- Al-Shammary AAK, Mohd Ma’amor NAA, Chen SQ, Lee KS, Mohd Hanafiah K. Bactericidal effects of in vitro 405 nm, 530 nm and 650 nm laser irradiation on methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Mycobacterium fortuitum. Lasers in Dental Science. 2020; 4: 111-121.
- Angarano V, Smet C, Akkermans S, Watt C, Chieffi A, Van Impe JFM. Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms. Antibiotics (Basel). 2020; 9.
- Plavskii VY, Mikulich AV, Tretyakova AI, Leusenka IA, Plavskaya LG, Kazyuchits OA, et al. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J Photochem Photobiol B. 2018; 183: 172-183.
- Hessling M, Wenzel U, Meurle T, Spellerberg B, Hönes K. Photoinactivation results of Enterococcus moraviensis with blue and violet light suggest the involvement of an unconsidered photosensitizer. Biochem Biophys Res Commun. 2020; 533: 813-817.
- Mohamad SA, Milward MR, Kuehne SA, Hadis MA, Palin WM, Cooper PR. Potential for direct application of blue light for photo-disinfection of dentine. Journal of Photochemistry and Photobiology B: Biology. 2021; 215.
- Uddin TM, Chakraborty AJ, Khusro A, Zidan BRM, Mitra S, Emran TB, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021; 14: 1750-1766.
- Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010; 130: 259-269.
Citation:Gal Hiltch, Cauana Oliva Tavares, José Antonio Poli de Figueiredo, Silvia Dias de Oliveira, Livia Ramos Alvariza, et al. Fluorescence Enhanced Theragnosis: A Principle that Serves to Educate and Provide Quality Assessment in Clinical Practice and Lab Training – Part A – Clinical Practice. J Dent & Oral Disord. 2025; 11(1): 1189.