Abstract
Nephronectin, an extracellular matrix protein also known as POEM, is considered to play critical roles in the development and functions of various tissues. This protein is associated with integrins, especially integrin a8β1, which are extracellular matrix receptors, through its own RGD motif, while interactions between nephronectin and integrin a8β1 are essential for early kidney development. The gene expression of nephronectin is regulated by various cytokines in osteoblasts, and enhances osteoblast differentiation and mineralization via EGF repeats and the 3’ UTR of its mRNA suggesting the important role of nephronectin in development and metabolism of bone. Nephronectin also plays important roles in vascularization of cardiac tissue, as well as the limbal and hair follicle stem cell niche. This review summarizes the rapidly evolving understanding of nephronectin, including protein and gene organization, expression and regulation, and effects both in vitro and . Recent novel findings have led to a new paradigm for matrix biology with major novel implications in regard to tissue development and morphogenesis.
Keywords: Nephronectin; Extracellular matrix protein; Kidney development; Osteoblast differentiation
Abbreviations
PCR: Polymerase Chain Reaction; EGF: Epidermal Growth Factor; ECM: Extra-Cellular Matrix; POEM: Pre-Osteoblast Epidermal Growth Factor-Like Repeat Protein with Meprin, A5 Protein, and Receptor Protein-Tyrosine Phosphatase μdomain; MAM: Meprin, A5 Protein, And Receptor Protein-Tyrosine Phosphatase μ; AP, ALP: Alkaline Phosphatase; ORF: Open Reading Frame; RGD: Arg-Gly- Asp; GDNF: Glia Cell-Line Derived Neurotrophic Factor; TGF-β: Transforming Growth Factor-β; TNF-a: Tumor Necrosis Factor-a; OSM: Oncostatin M; ERK: Extracellular Signal-Regulated Kinase; JNK: C-Jun N-Terminal Kinase; MAPK: Mitogen Activated Protein Kinase; NF-κB: Nuclear Factor Kappa B; UTR: Untranslated Region
Introduction
Extracellular matrix proteins are involved in a variety of biological functions, including cell growth, cell differentiation, and apoptosis. Recent findings have shown that in addition to the ability of extracellular molecules to communicate and interact with cells, specific interactions between extracellular molecules are essential for correct matrix organization and function [1]. Nephronectin, an extracellular matrix protein, was independently identified by two different groups at nearly the same time. Morimura et al. generated a pair of degenerate PCR primers based on EGF-like repeat structures found in various ECM and receptor proteins using single-strand cDNA from an osteoblast cell line (MC3T3-E1) as a template in order to isolate novel EGF-like repeats containing such genes as fibrillin and laminin [2]. Based on their results, a novel molecule termed preosteoblast Epidermal Growth Factor (EGF)-like repeat protein with meprin, A5 protein, and receptor Protein-Tyrosine Phosphatase μ domain (POEM) was reported. On the other hand, Brandenberger et al. sought to identify a ligand that mediates integrin a8β1 function during kidney morphogenesis in order to understand the mechanisms of integrin a8β1 function. They used soluble integrin a8β1-Alkaline Phosphatase (AP), which consisted of a heterodimer of the extra cellular domains of a8 and β1 fused to AP, in order to identify novel integrin a8β1-binding proteins in embryonic kidney extracts [3].
Nephronectin Protein Structures
Figure 1 shows the deduced nephronectin protein structure. A putative signal peptide representing the expected amino acid sequence is located at the NH2 terminus of the ORF of nephronectin, while no other hydrophobic regions serving as a transmembrane domain were found, suggesting that nephronectin is a secreted protein [4]. Nephronectin also contains five EGF-like repeats, a proline rich domain, and a region at the COOH terminus that shares homology with MAM repeats. It belongs to a group of small type EGF-repeat proteins that includes MAEG, Pref-1, EGFL6, Del1, DANCE, S1-5, DBI, and Spe-9, each of which play important roles in the functions and development of various organs [5-10]. Additionally, nephronectin contains an RGD (Arg-Gly-Asp) cell adhesion sequence motif. Sato et al. demonstrated that the LFEIFEIER (Lue-Phe-Glu- Ile-Phe-Glu-Ile-Glu-Arg) sequence on the COOH terminus of its RGD motif serves as a synergy site to ensure specific high-affinity binding of nephronectin to integrin a8 and β1 [11]. Nephronectin strongly binds to heparin, but not to Chondroitin Sulfate (CS)-E and moderately to heparan sulfate, while it fails to bind to CS-A, CS-C, CS-D, dermatan sulfate, and hyaluronic acid via its MAM domain [12]. Furthermore, Sanchez-Cortes et al. demonstrated that the FEI (Phe-Glu-Ile) motif in nephronectin can independently mediate cell adhesion of the RGD site, and both the RGD and FEI sequences in nephronectin synergistically bind to integrin a8β1 [13].
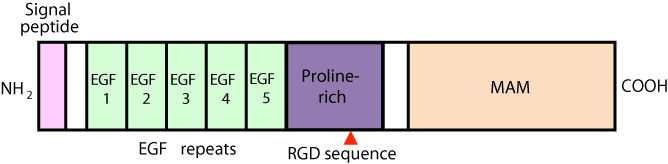
Figure 1: Structure of nephronectin protein. Nephronectin contains a signal
peptide, an array of five EGF repeats, an RGD (Arg-Gly-Asp) motif, and an
MAM domain.
Functions of Nephronectin
Nephronectin has been found to be expressed in various tissues and organs of embryonic day (E) 15.5 mice, such as the kidneys, lungs, choroid plexus, tongue, jawbone, dental epithelium, and facial bones (Figure 2) [2,3]. In addition, Kahai et al. noted that nephronectin was strongly expressed in the basement membrane of developing teeth and the extracellular matrix of developing jawbones [14]. In order to understand its physiological functions, Linton et al. generated a line of mice lacking the first exon of nephronectin (NpntΔex1; Npnt mutants) [15]. Npnt mutants were born at expected Mendelian frequency and appeared to be of normal size without any obvious external defects. However, they frequently displayed kidney agenesis and hypoplasia, which is caused by a delay in invasion of the metanephric mesenchyme in the ureteric bud at an early stage of kidney development [15]. In mice, kidney development begins on E7.0 when the ureteric bud, an outgrowth of the Wolffian duct, is induced to grow towards and invade the metanephric mesenchyme [16]. Furthermore, expression of GDNF, an essential growth factor in kidney development, was dramatically and specifically reduced in Npnt mutants in the metanephric mesenchyme at the time of ureteric bud invasion. These findings suggested that nephronectin promotes kidney development via integrin a8β1-mediated stimulation of GDNF expression [15]. The hair follicle bulge in the epidermis is associated with the Arrector Pili Muscle (APM), which is responsible for piloerection. Fujiwara et al. demonstrated that Npnt mutants have fewer arrector pili muscles formed in skin, which attach to the follicle above the bulge. Interestingly, compensatory up-regulation of the nephronectin family member EGFL6 was noted in hair follicle bulges of Npnt mutants, thus it is considered that bulge stem cells create a smooth muscle cell niche via nephronectin [17].
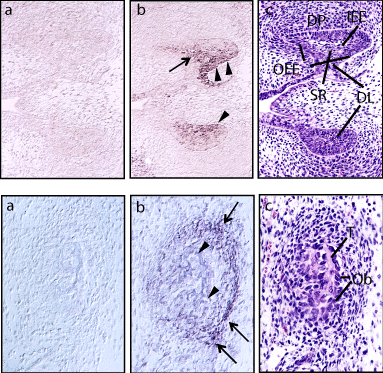
Figure 2: Nephronectin expression in molar teeth and ramus of mandible of
mouse obtained on E16.5. Nephronectin expression was noted in (A) molar
teeth and (B) ramus of the mandible. Frozen sections were hybridized with
a digoxigenin-labeled sense (a) or antisense (b) probe, or (C) stained with
hematoxylin and eosin. Expression in the Stellate Reticulum (SR) and outer
side of the Outer Enamel Epithelium (OEE) is indicated by an arrow and
arrowheads, respectively. DL: Dental Lamina; DP: Dental Papilla; IEE: Inner
Enamel Epithelium. Osteoblasts (Ob; arrowheads) lining the trabecular bone
(T) showed a low level and condensed mesenchymal cells (arrows) shoed a
relatively high level of nephronectin expression. Modified from Morimura et al.
[2] (https://www.jbc.org/content/276/45/42172.long).
Regulation of Nephronectin Gene Expression
To study the effects of several reagents that regulate osteoblast functions (Figure 2), MC3T3-E1 cells were established from newborn mouse calvaria and selected on the basis of high ALP activity in a confluent state. Those were found to constitutively express modest levels of nephronectin mRNA [2,18]. Miyazono et al. demonstrated that TGF-β known to inhibit the progression of osteoblast differentiation, suppressed nephronectin gene expression in both time- and dose-dependent manners via activation of the TGF-β receptor I and MAPK (ERK1/2 and JNK) pathways [19]. Interestingly, inhibition of a TGF-β type I receptor (also known as Alk5) by the specific inhibitor 2-[3-(6-methylpyridin-2-yl)-1H-pyrazol-4-yl]-1,5- naphthyridine strongly induced nephronectin expression, while that was also induced by introduction of an siRNA for Smad2, a central intracellular mediator of TGF-β signaling [20]. TNF-a known to be a key regulator of bone matrix properties and composition, blocks terminal osteoblast differentiation by inhibiting the expression of Runx2 and osterix, transcriptional factors essential for bone formation, which results in decreased expression of osteoblast-specific differentiation makers such as ALP and osteocalcin [21]. Tsukasaki et al. reported that TNF-a induced down-regulation of nephronectin gene expression in both time- and dose-dependent manners via the NF-κB pathway. In addition, down-regulation of nephronectin is known to influence the inhibition of osteoblast differentiation by TNF-a [22]. Oncostatin M (OSM) is a cytokine and member of the Interleukin (IL)-6 subfamily that includes IL-6, Leukemia Inhibitory Factor (LIF) and IL-11 [23]. Kurosawa et al. showed that OSM strongly inhibited nephronectin gene expression via the JAK/ STAT and MAPK (ERK1/2, p38 MAPK, JNK) pathways [24] (Figure 5). Furthermore, Sunadome et al. noted regulation of nephronectin expression by Klf transcription factors via ERK5 activation in C2C12 cells during skeletal muscle differentiation [25].
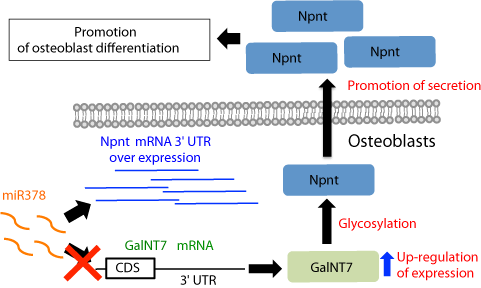
Figure 5: Regulation of nephronectin gene expression by TGF-β, TNF-a
and OSM. Nephronectin (Npnt) gene expression is inhibited by TGF-β via
the MAPK pathway, by TNF-a via the NF-κB pathway, and by OSM via the
JAK/STAT and MAPK pathways, while Npnt gene expression is enhanced
by Smad2.
Nephronectin Functions Related to Osteoblast Differentiation
To investigate its role during osteoblast differentiation (Figure 3), full-length nephronectin was expressed in MC3T3-E1 cells, which showed that osteoblast differentiation is enhanced by nephronectin via its EGF repeats and increased phosphorylation of ERK1/2 is essential for nephronectin-enhanced differentiation of osteoblasts [14]. Furthermore, Lee et al. found that expression of a fragment containing the 3’-untranslated region (3’-UTR) of nephronectin in osteoblasts promoted cell differentiation induced by activation of the Wnt signal pathway [26]. Also, microRNA (miR)-378 binds to and targets the 3’ UTR of nephronectin, which modulates osteoblast differentiation (Figure 4). Interestingly, this is the consequence of competition for miR-378 binding between nephronectin and UDP-N-acetyl-alpha- D-galactosamine: polypeptide-N-acetylgalactosaminyltransferase 7 (GalNT7) [27,28]. Also, Lee et al. demonstrated that several microRNAs (miR-23a, miR-101s, miR-296-5p, miR-328, miR-340- 3p, miR-425) bind to and directly target the nephronectin 3’ UTR, resulting in enhanced osteoblast differentiation [26,28].
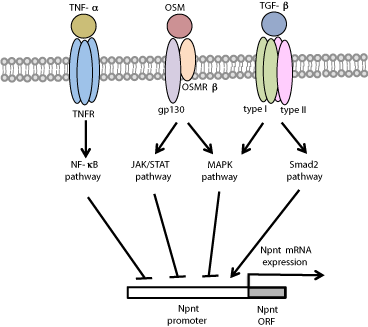
Figure 3: Regulation of osteoblast differentiation by nephronectin.
Nephronectin (Npnt) regulates osteoblast differentiation through its EGF
repeats. The receptor for EGF repeats has yet to be defined. Furthermore,
the effects of osteoblast differentiation on Npnt via integrin a8β1 have not
been identified.
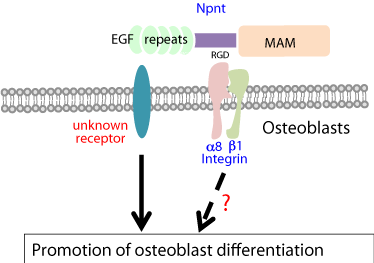
Figure 4: Nephronectin mRNA 3’ UTR modulates miR378 binding to 3’ UTR
of GalNT7 mRNA, and regulates nephronectin glycosylation and secretion.
Over-expression of nephronectin (Npnt) mRNA 3’ UTR inhibits miR378-
mediated GalNT7 degradation because of competition between Npnt and
GalNT7 for miR378 binding, resulting in increased GalN7 activity. This leads
to increased Npnt glycosylation and secretion, resulting in promotion of
osteoblast differentiation.
Diseases Associated with Nephronectin
Studies of diseases associated with nephronectin have recently been reported. Diabetic nephropathy, the most frequent cause of endstage renal disease, is characterized by development of progressive glomerulosclerosis and tubulointerstitial kidney lesions, which gradually lead to increasing loss of function of the kidney parenchyma [29]. Nephronectin was found to be highly expressed in glomeruli obtained from patients with diabetic nephropathy as compared to those with other kidney diseases [30]. Also, nephronectin was shown to be abundantly expressed in patients with a papillary thyroid carcinoma, the most common form of follicular cell-derived carcinoma, while it was also found to suppress tumor progression in cases of malignant melanoma [31,32]. Acute tubular necrosis is characterized by an initiation phase, followed by an extension phase, and then a maintenance and recovery phase, the latter of which involves increased regeneration of tubular cells [33]. Cheng et al. reported that nephronectin gene expression was enhanced during regeneration of tubular epithelium in a study that utilized an experimental model of acute tubular necrosis [34,35]. Fraser syndrome, caused by QBRICK dysfunction, is a rare autosomal recessive multi-organ disorder and characterized by cryptophthalmos, syndactyly, and renal agenesis, as well as a variety of morphogenetic defects [36]. Kiyozumi et al. demonstrated that QBRICK facilitates integrin a8β1-dependent interaction of cells with basement membranes by regulating the basement membrane assembly of nephronectin [37].
Conclusion
The present review provides a summary of current understanding of nephronectin, including its protein and gene organization, expression and regulation, and effects both in vitro and . Recently, nephronectin has been proposed to have excellent properties for cardiomyocyte adhesion and function, and indicated to have potential to improve current cardiac tissue engineering approaches for generation of cardiac patches [38]. Interestingly, osteopontin, which was initially regarded as an RGD-containing adhesive bone matrix protein and integrin a8β1-binding protein, also plays an important role in tooth formation, suggesting that nephronectin as well is critical for tooth development [39,40]. Although it has been clearly shown that nephronectin may play key roles in several aspects of tooth and craniofacial development, a more detailed mechanistic understanding of its varied roles is required for development of therapeutic approaches to suppress related disease pathogenesis.
Acknowledgment
We thank Drs. Morimura N., Miyazono A., Tsukasaki M., Aizawa R., Kurosawa T., and Hiranuma K. for their helpful comments. This work was supported in part by the Project to Establish Strategic Research Center for Innovative Dentistry by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
References
- Kielty CM, Shuttleworth CA. Fibrillin-containing microfibrils: structure and function in health and disease. Int J Biochem Cell Biol. 1995; 27: 747-760.
- Morimura N, Tezuka Y, Watanabe N, Yasuda M, Miyatani S, Hozumi N, et al. Molecular cloning of POEM: a novel adhesion molecule that interacts with alpha8beta1 integrin. J Biol Chem. 2001; 276: 42172-42181.
- Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, et al. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J cell biol. 2001; 154: 447-458.
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein eng. 1997; 10: 1-6.
- Osada A, Kiyozumi D, Tsutsui K, Ono Y, Weber CN, Sugimoto N, et al. Expression of MAEG, a novel basement membrane protein, in mouse hair follicle morphogenesis. Experimental cell research. 2005; 303: 148-159.
- Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993; 73: 725-734.
- Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998; 12: 21-33.
- Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999; 274: 22476-22483.
- Lecka-Czernik B, Lumpkin CK Jr, Goldstein S. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol cell Biol. 1995; 15: 120-128.
- Singson A, Mercer KB, L'Hernault SW. The C. elegans spe-9 gene encodes a sperm transmembrane protein that contains EGF-like repeats and is required for fertilization. Cell. 1998; 93: 71-79.
- Sato Y, Uemura T, Morimitsu K, Sato-Nishiuchi R, Manabe R, Takagi J, et al. Molecular basis of the recognition of nephronectin by integrin alpha8beta1. J Biol Chem. 2009; 284: 14524-14536.
- Sato Y, Shimono C, Li S, Nakano I, Norioka N, Sugiura N, et al. Nephronectin binds to heparan sulfate proteoglycans via its MAM domain. Matrix Biol. 2013; 32: 188-195.
- Sanchez-Cortes J, Mrksich M. Using self-assembled monolayers to understand alpha8beta1-mediated cell adhesion to RGD and FEI motifs in nephronectin. ACS Chem Biol. 2011; 6: 1078-1086.
- Kahai S, Lee SC, Seth A, Yang BB. Nephronectin promotes osteoblast differentiation via the epidermal growth factor-like repeats. FEBS Lett. 2010; 584: 233-238.
- Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development. 2007; 134: 2501-2509.
- Miner JH. Mystery solved: discovery of a novel integrin ligand in the developing kidney. J Cell Biol. 2001; 154: 257-259.
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011; 144: 577-589.
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. in vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983; 96: 191-198.
- Miyazono A, Yamada A, Morimura N, Takami M, Suzuki D, Kobayashi M, et al. TGF-beta suppresses POEM expression through ERK1/2 and JNK in osteoblasts. FEBS Lett. 2007; 581: 5321-5326.
- Tsukasaki M, Yamada A, Yoshimura K, Miyazono A, Yamamoto M, Takami M, et al. Nephronectin expression is regulated by SMAD signaling in osteoblast-like MC3T3-E1 cells. Biochem Biophys Res Commun. 2012; 425: 390-392.
- David JP, Schett G. TNF and bone. Curr Dir Autoimmun. 2010; 11: 135-144.
- Tsukasaki M, Yamada A, Suzuki D, Aizawa R, Miyazono A, Miyamoto Y, et al. Expression of POEM, a positive regulator of osteoblast differentiation, is suppressed by TNF-alpha. Biochem Biophys Res Commun. 2011; 410: 766-770.
- Jazayeri JA, Carroll GJ, Vernallis AB. Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol. 2010; 10: 1-8.
- Kurosawa T, Yamada A, Takami M, Suzuki D, Saito Y, Hiranuma K, et al. Expression of nephronectin is inhibited by oncostatin M via both JAK/STAT and MAPK pathways. FEBS Open Bio. 2015; 5: 303-307.
- Sunadome K, Yamamoto T, Ebisuya M, Kondoh K, Sehara-Fujisawa A, Nishida E. ERK5 regulates muscle cell fusion through Klf transcription factors. Dev Cell. 2011; 20: 192-205.
- Lee SC, Fang L, Wang CH, Kahai S, Deng Z, Yang BB. A non-coding transcript of nephronectin promotes osteoblast differentiation by modulating microRNA functions. FEBS Lett. 2011; 585: 2610-2616.
- Kahai S, Lee SC, Lee DY, Yang J, Li M, Wang CH, et al. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PloS one. 2009; 4: e7535.
- Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013; 32: 74-85.
- Blutke A. Opening a treasure chest: glomerular proteome analyses of formalin-fixed paraffin-embedded kidney tissue in the investigation of diabetic nephropathy. Nephrol Dial Transplant. 2012; 27: 1695-1698.
- Nakatani S, Ishimura E, Mori K, Fukumoto S, Yamano S, Wei M, et al. Nephronectin expression in glomeruli of renal biopsy specimens from various kidney diseases: nephronectin is expressed in the mesangial matrix expansion of diabetic nephropathy. Nephron Clin Pract. 2012; 122: 114-121.
- Ban Y, Yamamoto G, Takada M, Hayashi S, Ban Y, Shimizu K, et al. Proteomic profiling of thyroid papillary carcinoma. J Thyroid Res. 2012; 2012: 815079.
- Kuphal S, Wallner S, Bosserhoff AK. Loss of nephronectin promotes tumor progression in malignant melanoma. Cancer Sci. 2008; 99: 229-233.
- Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol. 1998; 18: 505-518.
- Cheng CW, Ka SM, Yang SM, Shui HA, Hung YW, Ho PC, et al. Nephronectin expression in nephrotoxic acute tubular necrosis. Nephrol Dial Transplant 2008; 23: 101-109.
- Cheng CW, Rifai A, Ka SM, Shui HA, Lin YF, Lee WH, et al. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney international. 2005; 68: 2694-2703.
- Slavotinek AM, Tifft CJ. Fraser syndrome and cryptophthalmos: review of the diagnostic criteria and evidence for phenotypic modules in complex malformation syndromes. J Med Genet. 2002; 39: 623-633.
- Kiyozumi D, Takeichi M, Nakano I, Sato Y, Fukuda T, Sekiguchi K. Basement membrane assembly of the integrin alpha8beta1 ligand nephronectin requires Fraser syndrome-associated proteins. J Cell Biol. 2012; 197: 677-689.
- Patra C, Ricciardi F, Engel FB. The functional properties of nephronectin: an adhesion molecule for cardiac tissue engineering. Biomaterials. 2012; 33: 4327-4335.
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine & growth factor Rev. 2008; 19: 333-345.
- Morishita M, Ono N, Miyai K, Nakagawa T, Hanyu R, Nagao M, et al. Osteopontin deficiency enhances parathyroid hormone/ parathyroid hormone related peptide receptor (PPR) signaling-induced alteration in tooth formation and odontoblastic morphology. Tissue Cell. 2011; 43: 196-200.
