
Special Article - Oral Cancer
J Dent & Oral Disord. 2016; 2(4): 1019.
Does Adjuvant Radiation Therapy Improve Outcomes in Pt1-2N0 Oral Tongue Squamous Cell Carcinoma Patients with Isolated Perineural Invasion?
Singareddy R¹*, Bajwa HK¹, Reddy MM², Raju AK³, Rao LMC4 and Rao TS5
¹Department of DNB Radiotherapy, Basavatarakam Indo American Cancer Hospital and Research Institute, India
²Department of Preventive and Social Medicine, Jawaharlal Institute of Post Graduate Medical Education and Research, India
³Department of Radiotherapy, Basavatarakam Indo American Cancer Hospital and Research Institute, India
4Head and Neck Oncology, Department of Surgical Oncology, Basavatarakam Indo American Cancer Hospital and Research Institute, India
5Department of Surgical Oncology, Basavatarakam Indo American Cancer Hospital and Research Institute, India
*Corresponding author: Singareddy R, Department of DNB Radiotherapy, Basavatarakam Indo American Cancer Hospital and Research Institute, India
Received: April 26, 2016; Accepted: May 27, 2016; Published: May 31, 2016
Abstract
Objectives: To assess the role of adjuvant radiation in pT1-2N0 Oral Tongue Squamous Cell Carcinoma (OTSCC) patients with isolated PNI for Locoregional Control (LRC) and Disease Free Survival (DFS)
Materials & Methods: We retrospectively reviewed hospital records from Jan 2012-Sep 2014 for pT1-2N0 OTSCC patients with isolated PNI. 40 patients were found among which 27(67.5%) received adjuvant radiation and 13(32.5%) did not. Univariate analysis was done to find significance between the recurrence and study variables using Fischer’s exact test. Kaplan-Meier analysis with logrank test was used for disease free survival
Results: Median follows up was 25 months. LRC for patients who received adjuvant radiation and who did not receive adjuvant radiation was 88.9% (2 local & 1 regional recurrence) and 76.9% (1 local & 2 regional recurrence) respectively. Of the 40 patients studied six (15%) had locoregional recurrence and all the patients who died had recurrence. Thus, in our study the overall mortality rate was equal to the locoregional recurrence rate (15%). There was no significant difference in DFS between two groups (p=0.365). Univariate analysis showed no statistical significance with any of the variables (age, gender, pathological grading of cancer, pathological staging of cancer, type of neck dissection and receiving radiation therapy)
Conclusion: The study showed no significant difference in locoregional control and disease free survival between patients who received adjuvant radiotherapy and those who did not receive adjuvant radiotherapy
Keywords: Radiation therapy; Oral tongue squamous cell carcinoma; Perineural invasion
Introduction
India contributes up to 7.8% of the global cancer burden and 8.33% of global cancer deaths [1]. Head and neck cancer is a major problem that occurs in Asia, especially in Indian subcontinent. Worldwide more than
200 000 new cases of head and cancers are diagnosed each year. About 40% of the head and neck cancer patients present during advanced stage of disease in developed countries, whereas it is >60% in developing countries like India [2]. This could have a bearing in the nature of treatment provided for these patients including the use of adjuvant radiation therapy. Among the head and neck cancer oral cavity cancer is the most common cancer in India. Overall, oral cavity cancer is the third most common type of cancer and accounts for more than 30% of all cancers in India [3].
In the oral cavity excluding lip, tongue constitutes the most common subsite for squamous cell carcinoma. In carcinoma of tongue, surgery is the preferred mode of treatment in early stage of disease [4]. In advanced stages, surgical resection followed by Radiotherapy (RT) with or without chemotherapy is performed, and it seems to be beneficial [5]. Likewise other head and neck cancers, Post Operative Radiation Therapy (PORT) is recommended for Oral Tongue Squamous Cell Carcinoma (OTSCC) patients with large primary tumors (T3, T4), with close or positive surgical margins, and evidence of Perineural Invasion (PNI), multiple positive nodes, or Extra Capsular Spread (ECS). Data is limited for high-risk features of recurrence and PORT in early-stage OTSCC. Furthermore, most of the studies reported have studied a mixed patient population with oral cavity cancer [6,7].
Because of the extremely low salvage rate of recurred oral tongue cancer, the proper extent and modality of initial treatment is very important [8]. Pathological stage I and stage II disease with sufficient clear resection margins is generally considered as low-risk and does not require PORT [9]. Perineural Invasion (PNI) has been classified as an intermediate risk factor for recurrence and decreased survival [10,11]. The presence of Lympho Vascular Invasion (LVI) or microscopic tumor foci in muscle increases the risk of recurrence and PORT should be considered. Tumor thickness, or alternative synonyms such as “depth of invasion” or “tumor depth”, has been consistently identified as a predictor for cervical lymph node metastasis [12]. Adjuvant therapy is not without risks and selecting the appropriate treatment regimen based on risk assessment, while maintaining optimal survival outcomes is vital to the overall management of patients with OTSCC. With this background, we tried to assess the role of adjuvant radiation in pT1-2N0 OTSCC patients with isolated PNI for Locoregional Control (LRC) and Disease Free Survival (DFS).
Materials and Methods
This was a retrospective cohort study based on review of medical records of OTSCC patients treated at a Tertiary Cancer Center (TCC), South India from January 2012 to September 2014. The study was done after obtaining approval from the institutional review board. Inclusion criteria are pT1-2N0 OTSCC patients with isolated PNI. Exclusion criteria are pT3-4, pathological node positive, margin positive, close margins and positive lympho vascular invasion. 410 patients diagnosed with OTSCC underwent upfront surgery during the study period; 203 patients are diagnosed as stage pT1-2N0; 40 patients of the 203 patients are diagnosed with pT1-2N0 with isolated PNI. All the patients who had a follow up period of at least six months from the time of first visit to the hospital were included in the study.
The patients were followed up post treatment at 6 weeks initially, then every 3 months for first 2 years and every 6 months till 5 years and yearly thereafter, to determine locoregional control and survival. A clinical examination is done at each visit. Imaging and/or biopsy was done if recurrence was clinically suspected.
Tumor staging was based on the pathology findings, according to the American Joint Committee on Cancer Staging System, 7th edition. In addition, the following variables were recorded: size, depth of the primary tumor invasion (tumor thickness), and grade of differentiation, status of resection margins, lympho vascular invasion, and peri neural invasion. To determine the status of resection margins, positive margin is defined as carcinoma in situ or as invasive carcinoma at the resection margin, close margin was defined when the distance from invasive tumor front to the resection margin was less than 5 mm, clear margin was defined when the distance from invasive tumor front that is 5 mm or more from the resected margin.
All patients received surgery for the primary site and neck. Resection of the primary site was grouped by the extent of the resection as wide local excision, hemiglossectomy and total glossectomy. The Type of neck dissection used was classified as supraomohyoid or modified radical neck dissection. As this was a retrospective study, the indication for RT was already determined by the individual treating physician after discussing with the patient. A dose of 60Gy in 30 fractions over 6 weeks at 2Gy per fraction and 5 fractions a week is delivered to all patients who received radiation therapy; 14 patients received radiation by conventional technique and the remaining 13 patients by Intensity Modulated Radiation Therapy (IMRT). Data collection and entry was done between January–April 2015 using a structured data capture instrument.
Data Entry and Statistical Analysis
Data was entered using Microsoft Excel 2010 and analysed using IBM SPSS version 20.0. Continuous variable like age was expressed using mean (SD). Overall mortality rate and recurrence rate was expressed as proportions. Different staging and grading of disease, type of neck dissection done and number receiving radiation therapy were expressed as proportions. Univariate analyses were done to find significance between the recurrence of disease and study variables using Fischer’s exact test. Kaplan-Meier analysis with log-rank test was done to check for the difference between disease free survival time between the two treatment groups.
Results
Among the 40 patients studied, the median (IQR) follow up time was 25 (15 to 32) months. The mean (SD) age was 45.1 (10.8) years and 30 (75%) were males. All of them showed an Eastern Cooperative Oncology Group (ECOG) performance status of either “0” or “1”. The pathological staging of the disease, details of the surgery undergone and also regarding the radiotherapy treatment are as mentioned in (Table 1).
Study Characteristics
Frequency, n (%)
Surgery details
Type of surgery
Wide excision
35 (87.5)
Hemi glossectomy
02 (5.0)
Total glossectomy
03 (7.5)
Neck dissection
Unilateral
31 (77.5)
Bilateral
09 (22.5)
Number of lymph nodes resected,
(median (IQR))
18 (11 to 63)
Pathological grading#
Well differentiated
11 (27.5)
Moderately differentiated
27 (67.5)
Poorly differentiated
02 (5.0)
Pathological staging#
T1N0
11 (27.5)
T2N0
29 (72.5)
Radiation therapy details
Postoperative radiation therapy (PORT)
Received
27 (67.5)
Not received
13 (32.5)
Radiation Technique
Conventional
14 (51.8)
Intensity Modulated Radiotherapy(IMRT)
13 (48.2)
#7th Edition American Joint Committee on Cancer Staging System.
Table 1: Details of the pathological staging, surgery undergone and treatment received in pT1-2N0 Oral Tongue Squamous Cell Carcinoma patients with isolated perineural invasion (N=40).
Of the 40 patients studied six (15%) had locoregional recurrence and all the patients who died had recurrence. Thus, in our study the overall mortality rate was equal to the locoregional recurrence rate (15%). Locoregional control for patients who received adjuvant radiation and who did not receive adjuvant radiation was 88.9% (2 local & 1 regional recurrence) and 76.9% (1 local & 2 regional recurrence) respectively (Table 2). Of the six patients who had locoregional recurrence, five died within three months of recurrence (and one patient survived for 13 months after recurrence). The minimum time to develop recurrence during the follow up period was three months and the maximum was 11 months.
TREATMENT
Total No
LOCAL
RECURRENCE
REGIONAL
RECURRENCE
DEATHS
NO RT
13
1(7.7%)
2(15.3%)
3(23%)
RT
27
2(7.4%)
1(3.7%)
3(11.1%)
TOTAL
40
3
3
6
Table 2: Recurrence patterns in patients.
Kaplan-Meir analysis with log-rank test showed that there was statistically no significant difference in the disease free survival time among patients who received PORT and those who did not receive (p = 0.365). The mean survival time was noted to be 35 months and 37 months in patients who did not receive PORT and who received PORT respectively (Figure 1).Univariate analysis done to find any association between locoregional recurrence of the disease and independent variables like age, gender, pathological grading of cancer, pathological staging of cancer, type of neck dissection done and receiving radiation therapy showed no statistical significance with any of the variables under study (Table 3).
Study characteristics
Total, n
Recurrence present, n (%)
p value*
Age (in years)
≤40
17
03 (17.6)
1
>40
23
03 (13.0)
Gender
Male
30
04 (13.3)
0.629
Female
10
02 (20.0)
Pathological grading#
Well differentiated
11
02 (18.2)
1
Moderately/poorly differentiated
29
04 (13.8)
Pathological staging#
T1N0
11
01 (9.1)
1
T2N0
29
05 (17.2)
Neck dissection
Unilateral
31
04 (12.9)
0.602
Bilateral
9
02 (22.2)
Postoperative radiation therapy (PORT)
Received
27
03 (11.1)
0.37
Not received
13
03 (23.1)
#7th Edition American Joint Committee on Cancer Staging System, *Fischer’s Exact test.
Table 3: Factors associated with loco-regional recurrence of the disease in pT1- 2N0 Oral Tongue Squamous Cell Carcinoma patients with isolated perineural invasion (N=40).
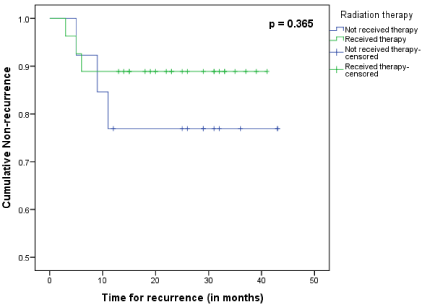
Figure 1: Kaplan-Meier curve for cumulative non recurrence in oral tongue
squamous cell carcinoma patients pT1-2N0 with isolated perineural invasion
who received radiation therapy compared to those who did not (N=40).
Discussion
It is well recognized that postoperative adjuvant RT in Oral Cavity Squamous Cell Carcinoma (OCSCC) patients may increase morbidity, chiefly arising from osteoradionecrosis, mucositis, and xerostomia. In addition, it is still unclear whether patients with pT1-2N0 disease benefit from adjuvant radiation in the presence of free margins and perineural invasion. Accordingly, perineural spread in head-andneck cancer is an infrequent but possibly aggressive manifestation of the disease [13-16]. Optimal management of postoperative RT for OCSCC patients with pT1-2N0 disease, perineural invasion, and tumor-free margins remains controversial [17-19]. This study was conducted to establish whether this group with isolated PNI would benefit from adjuvant radiation therapy in terms of local control and overall survival rate. In the present report, we deliberately excluded OCSCC patients with positive nodes, pT3 disease, or positive/close margins as these variables are all well-recognized independent prognosticators in subjects with head-and-neck cancer.
Several studies have sought to analyze the effect of PNI in OCSCC patients with N0 neck disease. Liao et al. analyzed 460 patients with clinical T1-3 and N0 neck disease; however 15% of their patients did not undergo neck dissections [20]. They found a significantly increased regional recurrence rate in the PNI+ group and found no benefit for their PNI+ patients undergoing adjuvant radiation treatment, similarly to our study.
In another study by Chinn et al, 88 OCSCC patients treated surgically with pN0 necks were studied. Overall 23% (20/88) were pN0/PNI+ and of those with PNI, 70% (14/20) underwent adjuvant radiotherapy. They concluded that PNI is an independent adverse risk factor in the absence of nodal metastasis and extracapsular spread. They observed a statistically significantly longer DFI and LRC when patients were treated with adjuvant radiation [21].
Our current findings clearly show that there is no significant differences in locoregional recurrence rate among patients with perineural invasion with the addition of adjuvant RT compared to patients with no adjuvant RT. Locoregional control for patients who received adjuvant radiation and who did not receive adjuvant radiation was 88.88% (2 local & 1 regional recurrence) and 76.92%(1 local & 2 regional recurrence) respectively. Independent variables like age, gender, pathological grading of cancer, pathological staging of cancer, type of neck dissection done and receiving radiation therapy showed no statistical significance for locoregional recurrence of the disease.
In our analysis altogether, our retrospective data supports the contention that surgical resection alone is sufficient treatment even for patients with perineural invasion if there is no other criteria to receive adjuvant therapy. In the interpretation of our findings, however, several limitations must be considered. First, our patient material is limited. Secondly the study is retrospective study and the discretion to use adjuvant radiotherapy has been in the hands of treating physician after discussing with the patient. Although we acknowledge the small sample size, this is one of the largest reviews of PNI as an isolated risk factor. Given the controversy in the literature regarding PNI as an absolute indication for adjuvant radiation, a larger randomized prospective trial would better answer the question of the role of adjuvant radiation for the pN0/PNI+ patient. In addition, better understanding of the molecular mechanisms for PNI is imperative when trying to identify high risk groups and to better understand the mechanism of perineural spread in HNSCC.
Conclusion
The study showed no significant difference in locoregional control and disease free survival between patients with PNI who received adjuvant radiotherapy and those who did not.
References
- Sarnath D, Khanna A. Current Status of Cancer Burden: Global and Indian Scenario. Biomed Res J. 2014; 1: 1-5
- Kulkarni MR. Head and Neck Cancer Burden in India. Int J Head Neck Surg. 2013; 4: 29-35
- Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006; 7: 108-112.
- Chen AY, Myers JN. Cancer of the oral cavity. Dis Mon. 2001; 47: 275-361.
- Fein DA, Mendenhall WM, Parsons JT, McCarty PJ, Stringer SP, Million RR, et al. Carcinoma of the oral tongue: a comparison of results and complications of treatment with radiotherapy and/or surgery. Head Neck. 1994; 16: 358-365.
- Lefebvre JL, Coche-Dequeant B, Buisset E, Mirabel X, Van JT, Prevost B. Management of early oral cavity cancer. Experience of Centre Oscar Lambret. Eur J Cancer B Oral Oncol. 1994; 30: 216-220.
- Lapeyre M, Bollet MA, Racadot S, Geoffrois L, Kaminsky MC, Hoffstetter S, et al. Postoperative brachytherapy alone and combined postoperative radiotherapy and brachytherapy boost for squamous cell carcinoma of the oral cavity, with positive or close margins. Head Neck. 2004; 26: 216-223.
- Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997; 19: 583-588.
- Brown JS, Shaw RJ, Bekiroglu F, Rogers SN. Systematic review of the current evidence in the use of postoperative radiotherapy for oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2012; 50: 481-489
- Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005; 27: 843-850.
- Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist. 2005; 10: 215-224.
- Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009; 115: 1489-1497.
- Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004; 26: 984-994.
- Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 423-431.
- McMahon J, O’Brien CJ, Pathak I, et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg. 2003; 41: 224-231.
- Vural E, Hutcheson J, Korourian S, Kechelava S, Hanna E. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000; 122: 717-720.
- Brown JS, Blackburn TK, Woolgar JA, Lowe D, Errington RD, Vaughan ED, et al. A comparison of outcomes for patients with oral squamous cell carcinoma at intermediate risk of recurrence treated by surgery alone or with post-operative radiotherapy. Oral Oncol. 2007; 43: 764-773.
- Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997; 39: 137-148.
- Langendijk JA, de Jong MA, Leemans CR, de Bree R, Smeele LE, Doornaert P, et al. Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: the importance of the overall treatment time. Int J Radiat Oncol Biol Phys. 2003; 57: 693-700.
- Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY. Does adjuvant radiation therapy improve outcomes in pT1-3N0 oral cavity cancer with tumor-free margins and perineural invasion? Int J Radiat Oncol Biol Phys. 2008; 71: 371-376.
- Chinn SB, Spector ME, Bellile EL, McHugh JB, Gernon TJ, Bradford CR, et al. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013; 149: 893-899.