
Special Article - Oral and Maxillofacial Surgery
J Dent & Oral Disord. 2016; 2(5): 1027.
Erythema Multiforme Associated with Herpes Simplex Virus: A Case Report and Literature Review
Davis K¹*, Smith C², Halpern L¹, Esuruoso O² and Ballard B³
¹Department of Oral and Maxillofacial Surgery, Meharry Medical College, USA
²Department of Internal Medicine, Meharry Medical College, USA
³Department of Pathology, Meharry Medical College, USA
*Corresponding author: Davis K, Department of Oral and Maxillofacial Surgery, Meharry Medical College, USA
Received: July 01, 2016; Accepted: July 18, 2016; Published: July 20, 2016
Abstract
Erythema Multiforme (EM) is an acute inflammatory skin disease where 90% of the minor cases follow outbreaks of herpes simplex. Clinical presentation entails the onset of macular, papular, urticarial, bullous, or purpuric symmetric lesions on extensor surfaces as well as oral mucous membrane involvement. Target lesions with clear centers and concentric erythematous rings may also be noted. This is a case report of HSV-1 associated EM in a 20 year-old female. The pathophysiology of disease presentation is discussed with a review of relevant literature, as well as, treatment options for resolution of EM triggered by HSV-1.
Keywords: Herpes simplex; Erythema multiforme; Infectious; Vesiculobullous; Immune-mediated; Stevens-Johnson syndrome
Abbreviations
AB: Antibody; SLE: Systemic Lupus Erythematosus; HSV: Herpes Simplex Virus; EM: Erythema Multiforme
Introduction
Erythema multiforme is an acute vesiculobullous, mucocutaneous disease that most often occurs concomitantly with exposure to infections or medications most often [1]. It presents with diverse mucocutaneous manifestations and is thought to be a type IV hypersensitivity reaction [1,2]. EM has often been linked to a subsequent exposure to the Herpes Simplex Virus (HSV-1) and is thought to be an immune mediated complication of the viral infection [3]. This is a case report of herpes simplex virus associated EM in a 20 year-old female. The pathophysiology of disease presentation is discussed with a review of relevant literature, as well as, treatment options for resolution of EM triggered by HSV-1.
Case Presentation
Present illness
A 20 year old African-American female presented to the Meharry Medical College Ambulatory Medicine Service complaining of “mouth sores and a rash.” The onset was noticed about 2 weeks prior to her seeking treatment. The patient stated sores were present to both her lips and intraorally with bleeding and pain noted. In addition, she described experiencing fatigue, headache, mild photophobia, sore throat, odynophagia, dysphagia, and mild arthralgias. About a week later, a pruritic rash had also developed involving her hands and elbows prompting her to obtain an emergency medicine evaluation at which time Clindamycin and Acyclovir were prescribed. She previously reported a “canker sore” about 6 months prior resulting in treatment for herpes with Acyclovir, but was also told at another visit that she didn’t have herpes despite noticing a tingling sensation prior to each “canker sore” presentation. The patient denied exposure to medications, food, or allergens that may have precipitated her symptoms.
Past medical history
The patient’s past medical history was unremarkable and there are no known drug allergies. A family history of Hypertension exists on her mother’s side. She denies ever having engaged in oral or vaginal sex as well as any history of tobacco, alcohol, or recreational drug use.
Physical Exam: The patient was febrile with a temperature of 100.1F. Clinical exam revealed tender and erythematous blisters of varying size on extensor and palmar surfaces of the hands (Figures 1 & 2). Violaceous plaques, macules, & blisters were also present to the forearms, and elbows (Figure 3). There were no target lesions on her back or lower extremities. Fundoscopic exam did not reveal any Roth spots or other significant findings suggesting autoimmune phenomena. Both the upper and lower lips were edematous with crusting & dried blood present that bled easily upon manipulation of scabs indicative of a vesiculobullous mechanism (Figure 4). Nikolsky sign was negative. Generalized erythema is visualized throughout the oral cavity. The buccal mucosa was markedly erythematous with hyperkeratotic & white plaque-like lesions present bilaterally. There were 1-2mm sized ulcerations present to both the hard and soft palate on erythematous bases. A palatal torus was present. The dorsal surface of the tongue had a white patch to plaque-type appearance that was not removable with a tongue blade as well as a large ulceration at the tip.
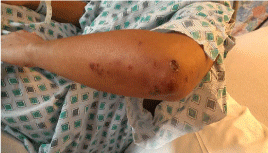
Figure 3: Purpuric lesions of elbow.
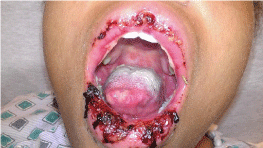
Figure 4: Necrotic hemorrhagic pseudomembrane of lips. Epithelial sloughing
with ulceration at tip of tongue.
Lab studies/Biopsy
The viral culture for herpes simplex was negative (likely secondary to patient’s late presentation and current antiviral use); however, serology for HSV IgG (chronic) AB I were positive and II negative. IgM (acute) AB for HSV I and II were both negative correlating with history of prior outbreaks. A lip bacterial wound culture ordered by the ED a week prior to her clinic presentation isolated Staph Aureus (MRSA) and Gram negative bacilli (Serratia marcescens) indicating bacterial superinfections with Clindamycin prescribed for treatment. Immunofluorescence was negative. Excisional biopsy of the buccal mucosa (hyperkeratotic lesion) performed by Oral and Maxillofacial Surgery histologically showed epidermal necrolysis with acute and chronic inflammatory cell infiltrates of the superficial dermis consistent with EM (Figure 5). In addition, the tissue punch biopsy performed by Internal Medicine of the elbow revealed basal cell hydropic degeneration and epidermal necrosis, also consistent with EM (Figures 6 & 7). All other laboratory values were within normal limits.
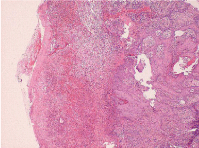
Figure 5: Buccal mucosa biopsy: Histologic examination reveals epidermal
necrolysis with acute and chronic inflammatory cell infiltrates of the superficial
dermis consistent with erythema multiforme. (H&E stain, magnification 4x).
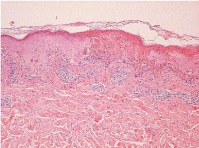
Figure 6: Punch biopsy elbow: Histologic examination reveals basal cell
hydropic degeneration and epidermal necrosis, consistent with erythema
multiforme. (H&E stain, magnification 10x).
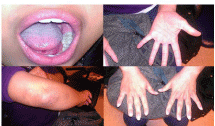
Figure 7: 5 months post treatment.
Differential diagnosis
The clinical presentation warrants consideration of other diagnoses including Behcet’s disease, Bullous Pemphigoid, Drug eruption, Contact Dermatitis, Sweet Syndrome (acute febrile neutrophilic dermatosis), Pemphigus Vulgaris, and Dermatitis Herpetiformis. Clinical features are described in (Table 1).
Clinical Features
Behcet’s Disease
Painful aphthous ulcers of mouth and genitals; cutaneous findings include erythema nodosum-like lesions, follicular rash & pathergy phenomenon (sterile pustule formation at site of needle stick)
Bullous Pemphigoid
Erosions of oral mucosa, bulla on erythematous; urticarial; or non-inflammatory base involving trunk, extremities, axillary & inguinal folds
Drug Eruption
Well demarcated, round macules & edematous plaques involving trunk & extremities usually sparing mucosal areas
Contact Dermatitis
Papular erythematous rash with indistinct margins
Sweet Syndrome (acute febrile neutrophilic dermatosis)
Painful cutaneous inflammatory papules, plaques, and nodules; oral ulcers, bullae, vesicles, gingival hyperplasia, necrotizing ulcerative periodontitis, and tongue swelling
Pemphigus Vulgaris
Mucosal blisters and erosions mainly affecting the buccal and palatal mucosa
Dermatitis Herpetiformis
Grouped pruritic papules and vesicles on extremities. Vesicles, erosions, or erythematous macules of oral mucosa or tongue
Table 1: Clinical features.
Work up/diagnosis
The Internal Medicine Service consulted Rheumatology and Infectious Disease for further evaluation to rule out any underlying autoimmune condition such as SLE or other infectious process. Rheumatology excluded the presence of any underlying autoimmune condition. Infectious Disease diagnosed recurrent stomatitis and extraoral lesions with recommendation to obtain quantitative immunoglobulin and immune electrophoresis. Dermatology consultation confirmed EM secondary to HSV-1.
Treatment
The Dermatology service recommended soaking of lips with sterile water for 20 minutes tid with gentle debridement of necrotic tissue & application of Vaseline as needed. Hospitalized treatment course consisted of Ciprofloxacin 400 mg IV every 12 hours, Vancomycin 1 g IV every 12 hours, Orabase (Triamcinolone 0.1%) to oral lesions every 8 hours prn, Viscous Lidocaine 15 ml swish & swallow tid prn, Morphine 1 mg every 4 hours prn, Acetaminophen 650mg prn every 4 hours on Day 1, the addition of Acyclovir 340 mg IV every 6 hours on Day 2, and Nystatin 500,000 units swish/spit every 6 hours and Hydroxyzine 25 mg po every 6 hours prn on Day 3. The patient was discharged on suppressive therapy with Valacyclovir 500mg po bid with a recommendation for one week follow up in the Infectious Disease clinic for immunologic testing. She subsequently failed to show for reevaluation; however, presented with a recurrence about a year later.
Discussion & Conclusion
The minor variation of EM typically causes the clinical manifestations associated with an HSV-I outbreak. One proposed pathogenesis involves Langerhans cells infected with HSV traveling to the epidermis and transferring viral DNA fragments to epidermal Keratinocytes [4]. HSV genes expressed in the skin leads to recruitment of HSV specific CD4+ T helper 1 cell that produce IFN (interferon) gamma in response to viral antigens. Release of IFN gamma initiates an inflammatory cascade that leads to epidermal damage and the inflammatory infiltrate that characterize cutaneous lesions, i.e.; macular, papular, urticarial, bullous, or purpuric symmetric lesions of EM on extensor surfaces as well as oral mucous membrane involvement [4]. Target lesions with clear centers and concentric erythematous rings may also be observed presenting within a wide spectrum of severity [5].
Diagnosis is often based upon patient history and clinical findings. The condition usually lasts 2-6 weeks and often reoccurs. The papules evolve into pathognomonic target lesions or iris lesions that appear within a 72-hour period and begin on the extremities. Lesions remain in a fixed location for at least 7 days and then begin to heal. They may also appear as arcuate lesions (Figures 1&2). Because this condition may be related to a persistent antigenic stimulus, recurrence is the rule rather than the exception, with most affected individuals experiencing 1-2 recurrences per year. Young adults are most frequently affected [6]. Mucosal involvement is present in as many as 70% of patients with erythema multiforme [7]. The degree is usually mild and limited to one mucosal surface. The most common sites of mucous membrane involvement in order of frequency are the oropharynx (lips, palate, and gingiva often affected), conjunctivae, genitalia, anus, tracheobronchial tree, esophagus, and bowel. Eye involvement (10%) is usually mild and may manifest as red conjunctivae, chemosis, and lacrimation. The genital areas may have painful, hemorrhagic bullae, and erosions. More severe erosions of at least 2 mucosal surfaces are seen in erythema multiforme major. Hemorrhagic crusting of the lips (25%) (Figure 4) and ulceration of the non-keratinized mucosa may also be seen. Occasionally, painful mucosal involvement may be extensive, with few or no skin lesions. Up to 50% of patients with Herpes Simplex Virus (HSV) associated erythema multiforme have oral ulcers.
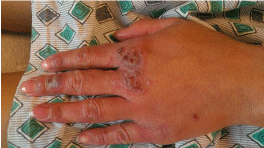
Figure 1: Target lesions to dorsal surface of hand.
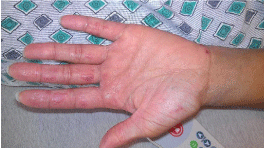
Figure 2: Target lesions noted to palmar surface of hand.
Erythema multiforme major and Steven-Johnson syndrome are more severe and potentially life-threatening disorders [8]. Lesions of Steven-Johnson syndrome typically begin on the face and trunk. They are flat, atypical lesions, described as irregular purpuric macules with occasional blistering. Most patients also have extensive mucosal involvement [9]. More than 50% of all cases are attributed to medications [10]. Controversy exists in the literature with regard to the clinical definitions of erythema multiforme major and Steven-Johnson syndrome and whether they are distinct entities or whether they represent a spectrum of one disease process [1,11,12]. International collaborators have suggested that erythema multiforme major and Steven-Johnson syndrome could be separated as two distinct clinical disorders with similar mucosal reactions but different patterns of cutaneous lesions. The syndrome was separated from the erythema multiforme spectrum and added to toxic epidermal necrolysis [13-15]. The two spectra are now divided into the following: (1) erythema multiforme consisting of erythema minor and major & (2) Steven-Johnson syndrome /Toxic Epidermal Necrolysis (SJS/ TEN).
The treatment course for HSV associated erythema multiforme depends on the severity and may include supportive measures such as systemic pain relief, analgesic mouth rinses, topical steroids, and antibacterial agents if secondary bacterial infections exist in addition to antivirals. Suppressive therapy with Acyclovir 400 mg bid for 6 months decreases the recurrence rate. Valacyclovir may be effective in cases unresponsive to Acyclovir.
References
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012; 51: 889-902.
- Prais D, Grisuru-Soen G, Barzilai A, Amir J. Varicella zoster virus infection associated with erythema multiforme in children. Infection. 2001; 29: 37–39.
- Samim F, Auluck A, Zed C, Williams PM. Erythema Multiforme: A Review of Epidemiology, Pathogenesis, Clinical Features, and Treatment. Dent Clin Noth AM. 2013; 57: 583-596.
- Wetter DA. Pathogenesis, clinical features, and diagnosis of erythema multiforme.
- McPhee SJ, Papadakis MA. Dermatologic Disorders. Berger TG, Rabow MW, editors. Current Medical Diagnosis & Treatment. 50th ed. New York: McGraw-Hill. 2011; 95-165.
- Marx R, Stern D. Immune-Based Disorders. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Vol 1. 2nd edn. Illinois: Quintessence Publishing. 2012; 143-210.
- Kempton J, Wright JM, Kerins C, Hale D. Misdiagnosis of Erythema Multiforme: A Literature Review and Case Report. Ped Dent. 2012; 34: 337-342.
- Cote B, Wechsler J, Bastuji-Garin S, Assier H, Revuz J, Roujeau JC. Clinicopathologic correlation in erythema multiforme and Stevens-Johnson syndrome. Arch Dermatol.1995; 131: 1268-1272.
- Ayangco LA, Rogers RS. Oral Manifestations of Erythema Multiforme. Dermatol Clin. 2003; 21: 195-205.
- Williams PM, Conklin R. Erythema multiforme: a review and contrast from Stevens-Johnson syndrome/toxic epidermal necrolysis. Dent Clin Noth AM. 2005; 49: 67-76.
- Assier H, Bastuji-Garin S, Revuz J, Roujeau JC. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995; 131: 539-543.
- Fritsch PO, Ruiz-Maldonado R. Erythema multiforme. Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Freedberg IM, Irwin M, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in General Medicine. 6th edn. New York: McGraw-Hill. 2003; 543-557.
- Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol. 1997; 24: 726-729.
- Weisman K, Petersen CS, Blichmann CW, Nielsen NH, Hultberg BM. Bullous erythema multiforme following herpes zoster and varicella-zoster virus infection. J Eur Acad Dermatol Venereol. 1998; 11: 147–150.
- Wollina U, Gemmeke A. Herpes zoster-associated erythema multiforme. J Dermatol Case Rep. 2009; 3: 11–13.