
Research Article
J Dent & Oral Disord. 2016; 2(7): 1035.
Design of Antimicrobial Release Systems Based on Chitosan and Copper Nanoparticles for Localized Periodontal Therapy
González JP¹, Covarrubias C¹*, Cádiz M¹, Corral C², Cuadra F¹, Fuentevilla I³ and Bittner M3,4
¹Laboratory of Nanobiomaterials, Institute for Research in Dental Sciences, Faculty of Dentistry, University of Chile, Chile
²Restorative Dentistry Department, Faculty of Dentistry, University of Chile, Chile
³Laboratorio de Microbiología Oral y Biotecnología, Faculty of Biological Sciences, University Andrés Bello, Chile
4Laboratorio de Microbiología Oral y Biotecnología, Faculty of Dentistry, Universidad Andrés Bello, Santiago, Chile
*Corresponding author: Covarrubias C, Laboratory of Nanobiomaterials, Institute for Research in Dental Sciences, Faculty of Dentistry, University of Chile, Santiago, Chile
Received: August 10, 2016; Accepted: September 08, 2016; Published: September 09, 2016
Abstract
Background: The aim of this study was to design an antimicrobial release system for periodontal therapy based on chitosan and copper nanoparticles and assess its in vitro antibacterial activity against Aggregatibacter actinomycetemcomitans.
Methods: Copper nanoparticles were synthesized into a chitosan, starch and ascorbic acid bio-friendly system. Copper nanoparticles/chitosan gel nanocomposites were used to produce solid sponges and gel spheres with 100 μg/mL copper content. The nanocomposite materials were characterized by scanning electron microscopy and attenuated total reflectance with Fourier transform infrared spectroscopy. The antimicrobial activity was tested against A. actinomycetemcomitans by halo inhibition assay on semisolid agar medium. Copper release from the nanocomposites was measured up to 10 days of incubation in artificial saliva at 37°C by analyzing the Cu concentration with a Copper Ion Selective Electrode.
Results: The formation of sponges and gel spheres of chitosan loaded with nanometric copper particles was confirmed. These materials inhibited the growth of A. Actinomycetemcomitans. Sphere nanocomposites presented higher stability in saliva and exhibited a controlled and sustained release of bactericidal copper concentrations.
Conclusions: Copper nanoparticles/chitosan nanocomposites effectively inhibit growth of A. Actinomycetemcomitans and appear as promissory systems for the development of localized periodontal therapies.
Keywords: Copper nanoparticles; Periodontal therapy; Aggregatibacter actinomycetemcomitans; Chitosan
Abbreviations
Cunp: Copper Nanoparticles; UV-Vis: Ultraviolet-Visible Spectrophotometry; SEM: Scanning Electron Microscopy; ATRFTIR: Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy; BS: Backscattering; EDX: Energy-Dispersive X-Ray Spectroscopy; ANOVA: Analysis of Variance; SD: Standard Deviation
Introduction
Periodontitis is one of the most common inflammatory diseases of humans, characterized by progressive destruction of the toothsupporting tissues; which can lead to the loss of teeth [1]. Although, the clinical distinction between chronic and aggressive periodontitis is not clear cut, one of the features of localized aggressive periodontitis is the relatively low level of gingival inflammation compared with chronic forms of periodontitis [2]. Elevated proportions of Aggregatibacter actinomycetemcomitans, and in some cases, of Porphyromonas gingivalis as well are commonly found in patients with aggressive periodontitis [3]. Aggregatibacter actinomycetemcomitans is a Gramnegative, nonmotile, facultative anaerobic cocobacillus bacterium that produces a variety of virulence factors, such as lipopolysaccharide, leukotoxin and Cytolethal Distending Toxin (CDT) and has been found be particularly more resistant to antibiotic treatment [4].
The current periodontal therapy is based on the mechanical removal of bacterial deposits from the root tooth surface [5] and on the use of systemic antibiotics with numerous side effects and development of bacterial resistance [6-8]. Several studies and recent reviews present metallic nanoparticles like silver and copper as a new generation of antimicrobial agents for biomedical applications [9- 13]. Both agents have proven efficacy as antimicrobials over a wide range of pathogens [14], however bactericide properties of copper can be achieved at much less cost than silver [15]. Although zerovalent Copper Nanoparticles (CuNP) exhibit a wide spectrum of antimicrobial activity against different species of microorganisms, including fungi and Gram-positive and Gram-negative bacteria, there are not reports about their activity against dental pathogens. The potential use of CuNP in localized antimicrobial therapies would require regulating the rate of activity in the site of action within an acceptable therapeutic time and concentration [16]. In the case of periodontal disease treatment, it is also desirable that the antimicrobial material have physical properties to be handled and molded into any desired shape and placed to the infected site [17]. Hydrogels such as chitosan have been used in transporting drugs with excellent results. Chitosan is a cationic amino polysaccharide obtained from chitin [18,19], and presents properties such as biodegradability, biocompatibility, non-toxicity, antimicrobial effects, hemostasis, bioadhesivity and it promotes drug absorption [20]. These properties and the ability of chitosan to form flexible physical presentations such as hydrogels, sponges, and microspheres, make it a viable option as a carrying matrix for CuNPs [18,19].
The synthesis of CuNPs is commonly carried out by using relatively toxic reducing agents [21,22], which are no compatible with medical applications. It has been previously optimized the synthesis of CuNP by using starch and ascorbic acid as more biocompatible chemical agents [23]. The appropriate incorporation of these bio-friendly nanoparticles into chitosan matrices could generate new antimicrobial systems for localized periodontal therapy. Chitosan gel doped with silver nanoparticles has demonstrated to have significantly higher antimicrobial activity against Escherichia coli than its components at their respective concentrations [24]. However, the preparation and antimicrobial activity of chitosan composite materials with CuNP have been not reported. Sponges and macrospheres of chitosan doped with CuNP could show better release properties compared to the fluid gel forms as they can behave as stronger and more resistance vehicles and adapt to different physical locations [18,19]
In this work the preparation of a new antimicrobial release systems based on CuNP-chitosan composites in the form of macrospheres and sponges with potential properties for localized periodontal therapy is presented. The antimicrobial activity of the composite is demonstrated against Aggregatibacter actinomycetemcomitans, pathogen often associated with aggressive periodontitis.
Materials and Methods
Preparation of CuNP/chitosan nanocomposites
Preparation of sponge nanocomposites: Sponge composites were produced from a CuNP/chitosan gel. For this purpose, 2.13 g of chitosan and 1.06 g of starch were dissolved in 73.2 mL of 10% ascorbic acid to obtain a viscous gel. Then, 838.4 μL of copper acetate 0.2 M (Cu(CH3COO)2) were added and heated in a microwave for 1 minute in two series of 30 seconds each. Thus, CuNP were in situ synthesized in the chitosan gel. After that, 26 mL of a 0.3% sodium polymetaphosphate /4% sodium hydroxide solution were added to obtain 100 mL of a 100 ppm CuNP/Chitosan crosslinked gel. Sponges were prepared individually placing 5 mL of CuNP/ chitosan crosslinked-gel into 24 well plates. The samples were frozen at -80º C for 24 hours and then freeze dried for 2-3 days to obtain sponge nanocomposites. Neat chitosan sponges were also prepared as control by using the same procedure and replacing the volume of CuNP suspension by distilled water.
Preparation of bead nanocomposites: Bead-shaped composites were prepared by adding 26 ml of distilled water to 73 mL of the CuNP/chitosan gel nanocomposite. Then, the gel was vertically dripped into a 100 mL of a 0.3% /sodium polymetaphosphate 4% NaOH solution by using a 10 mL syringe with a needle of 0.45 mm in diameter. Thus, nanocomposite beads of approximately 1.5 mm in diameter were produced and separated through a stainless steel sieve. Neat chitosan beads were also prepared as control following the same procedure and by using pure chitosan gel.
Synthesis of CuNP suspension
CuNP suspensions were used as control. The synthesis of CuNP was carried out following the procedure reported in a previous work [23]. Briefly, 0.242 g of starch was dissolved in 96.8 mL of ascorbic acid 10% for 1 min in a microwave (600 W). Then, 3.14 mL of copper acetate 0.2 M solution was added and heated in the microwave for 1 minute in two series of 30 seconds each. This procedure was used to obtain 100 ml of 400 ppm CuNP suspension.
Material characterization
The CuNP was identified through the surface plasmon resonance (a physical property of metallic nanoparticles) determined by Ultraviolet-Visible Spectrophotometry (UV-Vis). The size and morphology of CuNP were examined by scanning electron microscopy (SEM) with a Jeol JSM 5410 microscope. Chemical structure of the nanocomposite materials was characterized by Attenuated Total Reflectance with Fourier Transform Infrared Spectroscopy (ATR - FTIR) on an Agilent Cary 630 ATR-FTIR spectrometer. The microstructure of the sponge nanocomposite was characterized by SEM in Backscattering (BS) mode associated with Energy-Dispersive X-Ray Spectroscopy (EDX) for analysis of the chemical composition.
Antimicrobial activity
The antimicrobial activity of the CuNP/chitosan composites was tested against Aggregatibacter actinomycetemcomitans (serotype b). The strain was grown in BHI broth or agar (Brain Heart Infusion, Oxoid, Wesel, Germany) and incubated in a 5% CO2 atmosphere at 37°C for 48 h. From a grown plate, bacteria were transferred to a fresh BHI liquid medium, to a density equivalent to 0.5 McFarland. Then, 1 mL of the bacterial suspension was added to 7 mL of semi-solid BHI agar supported on 20 mL of solid medium. The antibacterial activity of the materials was assessed through the inhibition halo test. For this purpose, each sample (sponges, spheres, suspensions and controls) was individually placed into an approximately 2.5 cm³cavity created in the agar medium. After 48 h of incubation at 37°C in a 5% CO2 atmosphere, the diameters of the halos of bacterial growth inhibition were measured and photographed.
Cu release measurement
The release of Cu ions from the different composite materials was measured up to 10 days of incubation in artificial saliva pH 6.5 at 37°C [25]. Approximately 1 cm³ in volume of each material was immersed in 5 mL of artificial saliva. The Cu ion concentration in the aqueous phase was analyzed at different time intervals with a Copper Ion Selective Electrode (Hanna HI4115). The amount of copper released was calculated as copper mass per material volume (μg/cm³).
Statistical analysis
Statistical analysis of the mean values of the inhibition halos of each material was performed by one-way analysis of variance (ANOVA) followed by multiple comparison Tukey’s test. Statistical significance was set at p < 0.05. Each standard deviation (SD) serves as the estimate for the standard uncertainty associated with a particular measurement.
Results
The UV-vis absorption spectrum of the synthesized CuNP suspension displays a peak centered at 593 nm Figure 1, which correspond to the characteristic surface plasmon resonance of copper particles with nanometric dimensions. The size of the CuNPs, as estimated through SEM, was of approximately 90 nm.
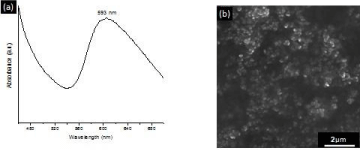
Figure 1: UV-Vis absorption spectrum of CuNP suspension displaying the
surface plasmon resonance at 593 nm (a). SEM image of the synthesized
CuNP (b).
The sponges and spheres produced from the CuNP/chitosan nanocomposite gel are presented in Figure 2. SEM-BS images of the sponge nanocomposite show an interconnected macroporous structure with pore size in the 4–10μm range. The presence of the CuNPs into the chitosan can be observed as bright spots on the material surface, which were identified as copper element through EDX analysis. The chemical structure of the neat chitosan and nanocomposite materials was also analyzed by ATR-FTIR Figure 3. Chitosan spectrum in the 400-2000 cm-1 range exhibit main vibration bands at 2,878, 1658-1606, ~1,358, and 1028 cm-1, corresponding to N-H/O-H, N-H, C-H and C-O bonds of the biopolymer structure, respectively. The N-H bands around 1650 cm-1 disappear in the sphere nanocomposite spectrum, whereas are slightly shifted to different wave numbers in the sponge nanocomposite.
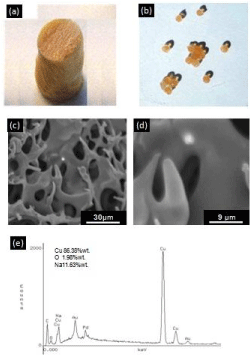
Figure 2: Photography’s of the sponge (a) and sphere (b) nanocomposites.
SEM-BS images of sponge nanocomposites revealing the presence of copper
into the chitosan matrix as bright spots (c-d). EDX compositional analysis of
the CuNP/chitosan nanocomposite.
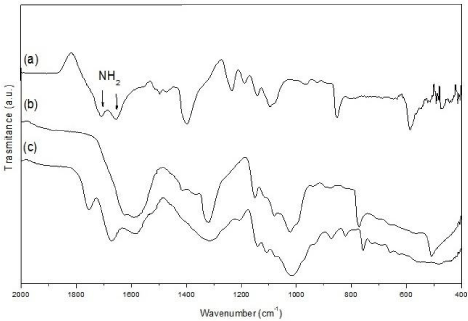
Figure 3: FTIR-ATR analysis of the neat chitosan (a), sponge (b) and sphere
(c) CuNP/chitosan nanocomposites.
The antimicrobial activity (indicated by the zones of inhibition) of the CuNP suspension, nanocomposites and neat chitosan materials is presented in Figures 4 and 5. The CuNP suspension, sponge and sphere nanocomposites presented a marked inhibition halo against A. Actinomycetemcomitans. The diameter of the inhibition zones of the sponge and sphere nanocomposites was significantly lower (p<0.05) with respect to the aqueous CuNP suspension, and the inhibition zone produced by the sponge was higher than that of the nanosphere. A relatively small inhibition zone was also detected in the neat chitosan materials, particularly in the chitosan spheres.
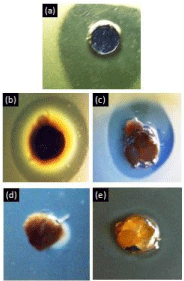
Figure 4: Inhibition halo of material samples incubated with A.
actinomycetemcomitans for 48 hours. 100 ppm CuNP aqueous suspension
(a), 100 ppm CuNP sponge nanocomposite (b), 100 ppm CuNP sphere
nanocomposite (c), Neat chitosan sponge (d), Neat chitosan sphere (e).
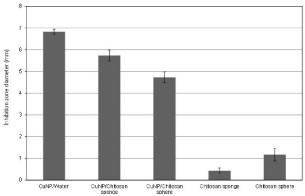
Figure 5: Inhibition zone diameter of the samples after 48 h of incubation with
A. actinomycetemcomitans.
The amount of Cu released per volume of nanocomposite material was measured in artificial saliva over a prolonged period of time Figure 6. The sponge system presented higher levels of Cu release than the spheres. However, the Cu release from sponge decreased after 3 days of incubation, whereas spheres exhibited a sustained Cu release, which was stabilized after 4 days at around 0.21 mg/cm³.
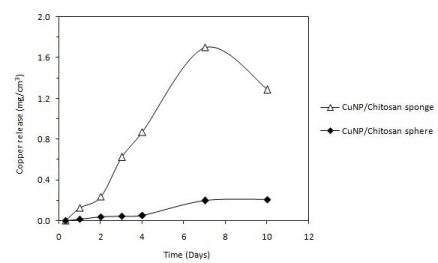
Figure 6: Copper release from sponge and sphere nanocomposites in artificial
saliva over a prolonged period of time. The amount of copper released is
expressed as copper mass per volume of nanocomposite material.
Discussion
Antibacterial delivery systems based on solid sponges and gel spheres of chitosan loaded with CuNPs were designed. Both CuNPs/Chitosan systems presented antibacterial activity against a representative pathogen of aggressive periodontitis. Although the antimicrobial activity of CuO against some oral pathogens has been previously reported [26] studies about zero-valent CuNPs against periodontal bacteria have not been found in the literature. In the current work, stable CuNPs aqueous suspension exhibited effect on the growth of A. actinomycetemcomitans. These periodontal bacteria have demonstrated to have relative resistance against traditional antibiotics such as metronidazole and amoxicillin [27] thus CuNPs could offer an alternative for the development of more effective periodontal therapies.
The inhibition mechanism of CuNPs on bacteria is generally explained by the effect of copper ions released from the metallic nanoparticle, which produce the rupture of the negatively charged bacterial cell wall, leading to protein denaturation and cell death [28]. More recently, Chatterjee et al. [29] demonstrated that the treatment of E. coli cells by CuNPs causes an increase in the level of Reactive Oxygen Species (ROS) in the cell bacteria, resulting in considerable lipid peroxidation, protein oxidation and DNA degradation, and finally the cell killing. Both, sponges and spheres of chitosan loaded with CuNPs were able to keep this antimicrobial effect. The smaller diameter of the inhibition zones produced by the nanocomposites indicates that the release of the CuNPs toward the bacterial medium takes place in more controlled manner. This effect can be explained by the slower diffusion of the nanoparticle due to physical and chemical interactions with the chitosan matrix. ATR-FTIR analysis showed that N-H bands of the chitosan structure are modified by the presence of the CuNPs, which suggest chemical interactions between chitosan and the metallic nanoparticles. In the case of the CuNPs/ chitosan sphere, N-H signals were completely absent, confirming a stronger interaction between chitosan N-H groups and the copper nanoparticle surface. This type of interactions together with the gradual degradation of the chitosan matrix affect the release behavior of the nanocomposites, producing a slower copper release from the sphere nanocomposite. In contrast, sponge nanocomposite exhibited low stability in artificial saliva, leading to a rapid release of the copper in the medium. The sphere nanocomposite kept a constant and prolonged release of copper over time, and the relatively low copper concentrations (0.21 mg/cm³) were found to be sufficient to produce the antibacterial effect. In addition to the antibacterial properties of the CuNPs, the antimicrobial activity of the CuNP/chitosan systems is also consequence of the well-known antimicrobial capability of the chitosan vehicle. It is generally accepted that positively charged amino groups of chitosan interact with the anionic parts of bacterial cell wall [30]. This electrostatic interaction results in changes in the properties of membrane wall permeability and hydrolysis of the peptidoglycans, leading to the leakage of intracellular components. Thus, the system based on CuNP/chitosan spheres presents antibacterial activity against A. actinomycetemcomitans and simultaneously exhibits a sustained release of copper over time. Furthermore, copper ions leached from metallic nanoparticle could also act on Matrix Metalloproteinases (MMPs), which degrade extracellular matrix components during periodontal destruction. Several enzymes involved in the metabolism of extracellular matrix components are known to be inhibited by excess of metal ions [31]. Copper ions have been found inhibit procollagen C/N proteinases, MMPs 2 and 9, which could thus regulate the periodontitis-associated collagen destruction [32].
In comparison with antibiotic - based localized release systems, the nanoparticle-based system could produce nanoparticle accumulation on the periodontal site due to the relatively low solubility of the metallic particle. So, in vivo animal experiments are required in the future in order to investigate the local and systemic distribution behavior of the CuNPs.
From the clinical point of view, a local delivery system can be considered as an extension of scaling and root planning in order to totally eliminate any residual infective/inflammatory component still harbouring in the periodontal apparatus. In addition, the delivery system can be an add-on supportive therapy in conjunction with systemic antibiotic therapy, which requires a prolonged availability of the antimicrobial agent in sufficient minimum inhibitory concentrations over a number of days. Thus, the properties exhibited by the nanocomposite gel spheres make of them attractive systems based on nanotechnology for the development of localized antimicrobial therapies of periodontal infections.
Conclusions
Local antimicrobial delivery systems based on CuNPs and chitosan were designed to nanoscale level. Both, sponge and sphere nanocomposite materials inhibit the growth of A. actinomycetemcomitans. However, sphere nanocomposites present higher stability in saliva and exhibit a more controlled and sustained release of bactericidal copper concentrations. The results of this work indicate that the CuNP/chitosan nanocomposites are promissory systems for the future development of localized periodontal therapies.
Acknowledgement
The authors acknowledge the support from U-Redes Project: Nanotechnology for Biomedical Applications Network (NanoBioMat) University of Chile.
References
- Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. J Periodontol. 2009; 80: 1021-1032.
- Agrawal N, Agarwal K, Varshney A, Agrawal N, Ashutosh Dubey. Is there a common pathogenesis in aggressive periodontitis & ankylosing spondylitis in HLA-B27 patient? Medical Hypotheses. 2016; 90: 63-65.
- Könönen E, Müller HP. Microbiology of aggressive periodontitis, Periodontology 2000. 2014; 65: 46-78.
- Oettinger-Barak O, Dashper SG, Catmull DV, Adams GG, Sela MN, Machtei EE, et al. Antibiotic susceptibility of aggregatibacter actinomycetemcomitans JP2 in a biofilm. J Oral Microbiol. 2013; 5: 20320.
- Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of Plaque-Induced Gingivitis, Chronic Periodontitis, and other clinical conditions. J Periodontol. 2001; 72: 1790-1800.
- Chadha VS, Arora K, Manjunath BC, Kalra S. Local drug delivery in periodontics: Current concepts and trends. IJAR. 2012; 1: 1-9.
- Slots J: Research, Science and Therapy Committee. Systemic antibiotics in periondontics. J Periodontol. 2004; 75: 1553-1565.
- Buchmann R, Müller RF, Van Dyke TE, Lange DE. Change of antibiotic susceptibility following periodontal therapy: A pilot study in aggresive periodontal disease. J Clin Periodontol. 2003; 30: 222-229.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008; 4: 707-716.
- Hernández-Sierra JF, Ruiz F, Pena DC, Martines-Gutierrez F, Martínez AE, Guillén A, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008; 4: 237–240.
- Valodkar M, Rathorea PS, Jadeja RN, Thounaojam M, Devkar RV, Thakore S. Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J Hazard Mater. 2012; 30: 201-202.
- Valodkar M, Modi S, Pal A, Thakore S. Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: A green approach. Mater Res Bull. 2011; 46: 384-389.
- Cioffi N, Ditaranto N, Torsi L, Picca RA, de Giglio E, Sabbatini L, et al. Synthesis, analytical characterization and bioactivity of Ag and Cu nanoparticles embedded in poly-vinyl-methyl-ketone films. Anal Bioanal Chem. 2005; 382: 1912-1918.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27: 76-83.
- Usman MS, Ibrahim NA, Shameli K, Zainuddin N, Yunus WM. Copper Nanoparticles Mediated by Chitosan: Synthesis and Characterization via Chemical Methods. Molecules. 2012; 17: 14928-14936.
- Greenstein G, Tonetti M. The Role of Controlled Drug Delivery for Periodontitis. The Research, Science and Therapy Committee of the American Academy of Periodontology. J Periodontol. 2000; 71: 125-140.
- Theivasanthi T, Alagar M. Studies of Copper Nanoparticles Effects On Micro-Organisms. Scholars Research Library. 2011; 2: 368-373.
- Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010; 62: 28-41.
- Herrera D, Alonso B, León R, Roldán S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol. 2008; 35: 45-66.
- Ruth HE. Chitosan: A biopolymer with drug delivery system applications. Madrid: Complutense University of Madrid, 2010. MDS thesis.
- Chen M, Wang LY, Han JT, Zhang JY, Li ZY, Qian DJ. Preparation and study of polyacryamide-stabilized silver nanoparticles through a one-pot process. J Phys Chem B. 2006; 110: 11224-11231.
- Lou XY, Yuan CL, Archer LA. An unusual example of hyperbranched metal nanocrystals and their shape evolution. Chem Mater. 2006; 18: 3921-3923.
- González JP. Synthesis of antibacterial materials based on metallic nanoparticles and biopolymers for periodontal therapy. Santiago: University of Chile, 2013. DDS thesis.
- Sanpui P, Murugadoss A, Prasad PV, Ghosh SS, Chattopadhyay A. The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int J Food Microbiol. 2008; 124: 142-146.
- Viennot S, Lissac M, Malquarti G, Dalard F, Grosgogeat B. Influence of casting procedures on the corrosion resistance of clinical dental alloys containing palladium. Acta Biomater. 2006; 2: 321-330.
- Vargas-Reus MA, Memarzadeha K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int J Antimicrob Agents. 2012; 40: 135-139.
- Ardila CM, López MA, Guzmán IC. High drug resistance of periodontopathogens in isolates of periodontal disease. Med Oral Patol Oral Cir Bucal. 2010; 15: 947-951.
- Lin YE, Vidic RD, Stout JE, McCartney CA, Yu VL. Inactivation of Mycobacterium Avium by Copper Silver Ions. Water Res. 1998; 32: 1997-2000.
- Chatterjee AK, Chakraborty R, Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014; 25: 135101.
- Goy RC, de Britto D, Assis O. A review of the Antimicrobial Activity of Chitosan, Polimeros. 2009; 19: 241-247.
- Gerlach RF, Souza AP, Cury JA, Line SRP. Effect of lead, cadmium and zinc onthe activity of enamel matrix proteinases in vitro. Eur J Oral Sci. 2000; 108: 327-334.
- de Souza AP, Gerlach RF, Line SR. Inhibition of human gingival gelatinases (MMP-2 and MMP-9) by metal salts. Dent Mater. 2000; 16:103-108.