
Special Article – Periodontics
J Dent & Oral Disord. 2016; 2(7): 1038.
Human Amniotic Membrane Graft used in Vestibuloplasty: A Case Report
Bansal N¹* and Gupta ND²
¹Department of Periodontics & Implantology, Divya Jyoti College of Dental Sciences & Research, India
²Department of Periodontics & Community Dentistry, Dr Ziauddin Ahmad Dental College (AMU), India
*Corresponding author: Neha Bansal, Department of Periodontics & Implantology, Divya Jyoti College of Dental Sciences & Research, Uttar Pradesh, India
Received: August 29, 2016; Accepted: October 16, 2016; Published: October 18, 2016
Abstract
Various grafting materials have been used for vestibuloplasty but all have met with variable results. Skin and mucosal grafts have disadvantages of need of second surgical site, limited amount of mucosa available for grafting, postoperative pain and risk of surgical complications at donor site. In contrast, Human Amniotic Membrane (HAM) does not have limitations as conventional grafts. Its several unique properties forge it as a suitable substitute for conventional skin and mucosal grafts. Aim of this article is to present a case report on treatment for vestibuloplasty using an amniotic membrane graft. We concluded that amniotic membrane can be used successfully as a mucosal graft substitute.
Keywords: Amnion; Vestibuloplasty; Graft; Atrophic mandible
Introduction
Facial and oral rehabilitation of patients with extreme mandibular atrophy has always remained a challenging task and been attempted with variety of treatment procedures such as ridge augmentation and vestibuloplasty. Vestibuloplasty is a mucogingival procedure designed to restore alveolar ridge height and to increase the amount of attached gingiva and vestibular depth by lowering muscles attached to the buccal, labial, and lingual aspects of the jaws [1]. One of the major challenges after a vestibuloplasty procedure is to reduce post operative discomfort, scar contracture and subsequent loss in sulcular depth. A raw bony surface, as is obtained after Clark’s vestibuloplasty is vulnerable to infections, increased pain and scarring during the healing phase. Skin and mucosal grafts are most commonly used to cover the exposed periosteal surface; however they have drawbacks as limited amount of mucosa available for grafting, need of second surgical site, postoperative pain and risk of surgical complications at donor site [2]. There is a constant search for biocompatible membranes/materials which would satisfy most criteria required of a biological scaffold.
Human Amniotic Membrane (HAM) is the innermost layer of placenta and histologically resembles the skin. Guler et al. elaborated superiority of HAM as a graft over other graft materials [3]. The human amnion membrane is a biological graft which has unique properties like wound protection, healing promoter, bacteriostatic, pain reduction, antiscarring and epithelization effects. It may be a graft of choice due to its easy availability and low cost [4]. Here we present a case of human amniotic membrane used as a graft in Vestibuloplasty and discuss biological characteristics of amniotic membrane which most likely make it an ideal graft material.
Case Presentation
Clinical presentation
Sixty years old female patient was referred for treatment at Department of Periodontics and Implantology, Dr Z. A. Dental College, Aligarh with chief complains of instability of lower denture during functions as speech and chewing. The patient’s medical history was non-contributory. She informed of wearing full dentures for the last 10 years; dentures were ill adapted. Bone height and mucosal quality were assessed using radiographic and clinical methods. Patient was having Stage 4 atrophic mandible (Cawood & Howell) [5] with reduced bone height and obliteration of the buccal and the lingual sulcus. Mucosa was thin, healthy pink in color with reduced muscle tone. No bony prominence or undercut was noticed on palpation of ridge. Therefore, to increase the denture supporting area, we planned to go for vestibuloplasty along with amnion grafting followed by new denture fabrication. The procedure to be performed was explained to patient, followed by informed written consent & institutional ethical committee clearance.
Preoperative impression, cast and measurements were made (Figure 1a&1b). The cast was arbitrarily scraped till the desired depth. A splint was fabricated with clear acrylic (Figure 1c).
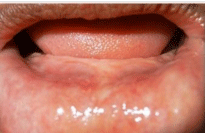
Figure 1a: a) Preoperative view showing atrophied mandible and decreased
vestibular depth.
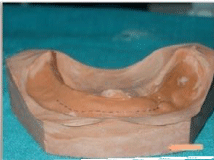
Figure 1b: b) Preoperative cast with markings representing decreased
vestibular depth.
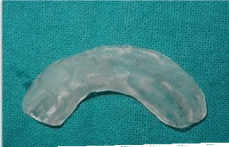
Figure 1c: c) Acrylic stent.
Amniotic membrane harvesting
Fresh amniotic membrane was obtained from placenta of healthy seronegative mothers. Donor was screened for hepatitis B and C, syphilis and human immunodeficiency virus. Amniotic membrane was prepared a day before the procedure by separating it from chorion of placenta under sterile aseptic conditions. Separated amnion was flushed with copious amounts of saline to clean any blood. Amnion was processed under sterile conditions using an antibiotic mixture comprising 400 ml of saline containing 1,200,000 IU benzathine penicillin and 100 ml of metronidazole to cover Gramnegative bacteria, Gram-positive bacteria and fungi was used as a decontaminant and storage medium.
Preservation
The membrane was then stored in a large bottle containing 400 ml of saline containing 1,200,000 IU benzathine penicillin at 4°C for 24 hr. On the day of application it was soaked in normal saline for 10 min.
Vestibuloplasty
Vestibular deepening was done using Clark’s technique (Figure 2a). After applying anesthesia, a horizontal mucosal incision in the mucogingival line was carried out. Mucosal flap was dissected from the lip base. The submucosal connective tissue and muscle attachment were incised, separated with a periosteal elevator from the periosteum and pushed apically. The separated mucosal flap was sutured to periosteum at desired depth (Figure 2b). The prepared amniotic graft material was transferred over the exposed periosteal wound surface area with mesenchymal/chorionic side against the exposed periosteum and sutured in place with 5-0 sutures (Figure 2c). The surgical splint was placed after lining with Coe pack (GC, Japan) to prevent formation of dead space, and secured with sutures.
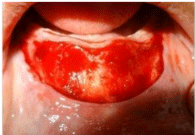
Figure 2a: a) Incision given in mucogingival line and partial thickness
dissection along with muscle attachment done.
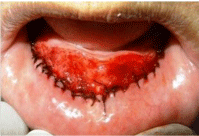
Figure 2b: b) Separated mucosal flap was sutured to periosteum at desired
depth.
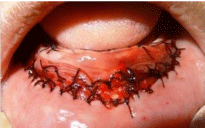
Figure 2c: c) Amniotic membrane placed & secured in place by suturing.
Follow up
The splint and sutures were removed 7th day postoperatively and grafted site was thoroughly cleaned with betadine solution. A hyperaemic reddish white necrotic soft tissue layer could be seen with underlying hyperemic tissue. By the end of the 4th week, the necrotic layer had disappeared, leaving slightly hyperemic mucosal tissue. Amnion had completely degenerated and disappeared (Figure 3). The patient complained of very little discomfort and mild pain with no burning sensation. There was minimal scarring. No other complications such as infection or graft rejection were observed. After 6 weeks, the patient was referred to the department of Prosthodontics for prosthetic rehabilitation.
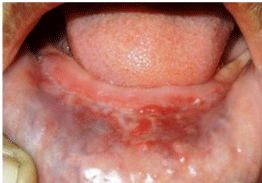
Figure 3: Post-operatively (one month)- there has been gain in vestibular
depth and increase in denture bearing area.
Discussion
An ideal scaffold/graft should be biocompatible, non toxic, non carcinogenic and non immunologenic. In addition the material should have adequate physical/mechanical properties like stability, elasticity, flexibility and appropriate resorbability at a rate congruent with tissue replacement. They should also allow for cellular adhesion and subsequent structural integrity [6].
Amniotic membrane is the innermost layer of developing embryo lying next to chorion. It contains almost all the qualities of an ideal dressing and has been used extensively as graft or surgical wound dressing in cases of burns, ocular surgeries, surgical reconstruction of the bladder and vagina and in the prevention of surgical adhesions. Harvesting the HAM is a simple procedure and does not require any special arrangements. Different preservation methods has been used including cryopreservation, storage in antibiotic solution, preservation in silver nitrate, glycerol-preserved sheets, dried sheets and gamma irradiated sheets [7].
Its unique combination of properties makes it a suitable graft material. Gupta et al. [8] reviewed the properties and potential applications of amniotic membrane in periodontology. They described that amniotic membrane serves as a scaffold that easily integrates with host tissue and contains numerous angiogenic & growth factors which provide an excellent environment for cell migration and differentiation, promoting wound healing and rapid re-epithelialization. It contains thrombospondin 1 which promote neovascularization. It also contains epidermal growth factor, Transforming Growth Factor (TGF) beta, fibroblast growth factors, platelet-derived growth factors, metalloproteinases, and tissue inhibitors of matrix metalloproteins. These regenerative molecules impart wound healing properties to HAM. Unlike other synthetic materials, it provides sufficient oxygenation for epithelial and other cells because of its good permeability. It inhibits scarring by down regulating Transforming Growth Factor β (TGF) and its receptor expression by fibroblasts.
It has antimicrobial, antiviral and anti-inflammatory properties [9]. It acts as selective barrier membrane; reduces influx of inflammatory cells and mediators at the site of surgery. Kjaergaard et al. have shown in vitro antimicrobial effects of the amnion and chorion against certain microorganisms [10]. It secretes various antimicrobial peptides such as β-defensins and cystatin E. These molecules confer antibacterial and antiviral abilities to HAM respectively. There is still further need for studies to verify these properties of the amniotic membrane. The collagen fibers of amniotic basement membrane contribute to hemostatic attribute of HAM; this minimizes bleeding and hematoma formation. Also, HAM gets adhered to underlying wound surface and prevents dead space formation. This diminishes the accumulation of microbes and serous discharge.
One of important property of amniotic membrane is that it is Non immunogenic. Amniotic cells do not express HLA-A and B class I MHC antigens making it ‘immunologically privileged’; Hence HAM shows no graft rejection [11].
Amniotic membrane may be infected by normal vaginal flora, herpes, chlamydia or other contaminant bacteria. In addition there is risk of transmission of viruses like HIV, Hepatitis, syphilis and others. To prevent the chances of cross infection, donor mother should be properly screened and proper sterilization modalities need to be instituted [12]. The other issue of concern is ethical clearance involved in such procedure, so informed consent should be taken from patient.
Our results supports the findings of other studies showing that amniotic membrane can be successfully used as a graft in mucogingival surgeries [4,13-15].
Conclusion
Based on clinical case described we can conclude that human amniotic membrane can be used as a successful and viable graft for covering of the raw periosteal surface, preventing secondary contraction after vestibuloplasty, and maintaining the postoperative vestibular depth. However, further studies are needed to confirm its advantages and usage in the future.
References
- Mosby Dental dictionary.
- James R. Hupp, Myron R. Tucker, Edward Ellis III. Contemporary Oral and Maxillofacial Surgery. 6th edn. Mosby, 2013: 226-231.
- Guler R, Ercan MT, Ulutuncel N, Devrim H, Uran N. Measurement of blood flow by the 133Xe clearance technique to grafts of amnion used in vestibuloplasty. Br J Oral Maxillofac Surg. 1997; 35: 280-283.
- Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty: A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 574-578.
- Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988; 17: 232-236.
- Niknejad H, Peirovi H. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008; 15: 88-99.
- Halim AS, Khoo TL, Mohd. Yussof SJ. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg. 2010; 43: 23-28.
- Gupta A, Kedige Sd, Jain K. Amnion and Chorion Membranes: Potential Stem Cell Reservoir with Wide Applications in Periodontics. Int J Biomater. 2015; 274082.
- Sangwan VS, Burman S, Tejwani S, Mahesh SP, Murthy R. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007; 55: 251-260.
- Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schønheyder HC, Uldbjerg N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynaecol Reprod Biol. 2001; 94: 224-229.
- Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981; 318: 1003-1005.
- Qureshi IZ, Fareeha A, Khan WA. Technique for processing and preservation of human amniotic membrane for ocular surface reconstruction. World Acad Sci Eng Technol. 2010; 69: 763-766.
- Kothari CR, Goudar G, Hallur N, Kothari MC. Use of amnion as a graft material in vestibuloplasty: A clinical study. Br J Oral Maxillofac Surg. 2011; 50: 545-549.
- Sikkerimath BC, Dandagi S, Gudi SS, Jayapalan D. Comparison of vestibular sulcus depth in vestibuloplasty using standard Clark's technique with and without amnion as graft material. Ann Maxillofac Surg. 2012; 2: 30-35.
- Keerthi R, Vaibhav N, Raut R. Amniotic Membrane as a Biological Scaffold After Vestibuloplasty. J Maxillofac Oral Surg. 2015; 14: 383-387.