
Special Article - Periodontics
J Dent & Oral Disord. 2017; 3(2): 1057.
Unrevealed Mechanisms of Bacterial Virulence in Periodontitis and Peri-Implantitis – Gingipains
Trivedi AR*, Jathal BS, Patel VG, Gupta SA, Purani HJ, Sahayata VN and Nayak V
Department of Periodontology and Implantology, Faculty of Dental Sciences, Dharmsinh Desai University, India
*Corresponding author: Trivedi AR, Department of Periodontology and Implantology, Faculty of Dental Sciences, Dharmsinh Desai University, India
Received: March 01, 2017; Accepted: April 11, 2017; Published: April 18, 2017
Abstract
As a part of dental plaque biofilm, Porphyromonas gingivalis is a major causative bacterium of chronic and aggressive periodontitis and play major role in development of peri-implant mucositits and peri-implantitis also. Arginine and lysine-specific proteases- gingipains HRgpA, RgpB and Kgp produced by P. gingivalis are fatal virulence factors for damaging host tissues. Periodontitis and peri-implantitis sites are both inflammatory lesions initiated by a bacterial infection and exist in a non-sterile environment with common cellular components and molecular pathways. These observations suggest that host modulatory approaches like inhibition of gingipains by various methods; those are successful for periodontitis will likely be applicable to peri-implantitis. For the reason, it is important to understand the structural and functional characteristics of gingipains. Present review is an attempt to explain these characteristics, which may be helpful in development of newer treatment strategies.
Keywords: HRgpA; RgpB; Kgp; Periodontitis; Peri-implantitis
Introduction
Chronic periodontitis is an infectious disease resulting in inflammation with in supporting tissues of the teeth, progressive attachment loss and bone loss. Generalized Aggressive Periodontitis (GAgP) is a chronic inflammatory disease of the periodontium, which is characterized by an accelerated rate of generalized alveolar bone resorption and, in severe cases, can lead to early tooth loss [1]. The term peri-implantitis was introduced in the 1980s to describe a destructive inflammatory process affecting the soft and hard tissues around osseointegrated implants, leading to the formation of a periimplant pocket and loss of supporting bone (1st European Workshop on Periodontology, Ittingen, Switzerland). Despite rigorous clinical intervention strategies, recent reports suggest that up to 30% of adults in the U. S. over the age of 40 years have measurable periodontal bone loss [2]. Numerous studies implicate a variety of organisms with periodontal disease; however, Porphyromonas gingivalis remains the preeminent organism associated with periodontal and peri-implant diseases [3].
Gingipains are trypsin-like cysteine proteinases produced by Porphyromonas gingivalis. The rgpA gene of Porphyromonas gingivalis encodes the isoforms of the arginine-specific proteases HRgpA and RgpA(cat) and the membrane type mt-RgpA (cat), while rgpB encodes RgpB and the membrane type mt-RgpB. The lysinespecific protease Kgp is encoded by the kgp gene. Rgps (HRgpA and RgpB) and Kgp are specific for -Arg-Xaa- and -Lys-Xaa- peptide bonds, respectively [3].
Porphyromonas gingivalis derived gingipains play mojor role directly and indirectly at every stage of infection, not only in chronic and aggressive periodontitis, but also in occurrence of periimplant mucositis and peri-implantitis by microbial attachment and colonization, acquisition of nutrients, evasion of host defence, and tissue invasion and dissemination suggested by Prof. Marzena Dominiak at FDI Annual world Dental Congress-2016 in Poznan, Poland.
Rgps enhance vascular permeability through prekallikrein activation or direct bradykinin release in combination with Kgp. This Rgp action is potentially associated with gingival edema and crevicular fluid production. Rgps activate the blood coagulation system, leading to progression of inflammation and consequent alveolar bone loss in the periodontitis site [4]. Rgps also activate protease-activated receptors and induce platelet aggregation, which, together with the coagulation-inducing activity, may explain an emerging link between periodontitis and cardiovascular disease [4,5].
Kgp is the most potent fibrinogen/fibrin degrading enzyme of the three gingipains in human plasma, being involved in the bleeding tendency at the diseased gingiva. Gingipains stimulate expression of Matrix Metalloproteinases (MMPs) in fibroblasts and activate secreted latent MMPs that can destroy periodontal tissues. Gingipains degrade cytokines, components of the complement system and several receptors, including macrophage CD14, T cell CD4 and CD8, thus perturbing the host-defense systems and thereby facilitating sustained colonization of P. gingivalis. Gingipains are potent virulence factors of P. gingivalis, and in many regards their pathogenic activities constitute new mechanisms of bacterial virulence [6-8].
Structural characterization of Rgps and Kgp [9-13] (Figure 1,2).
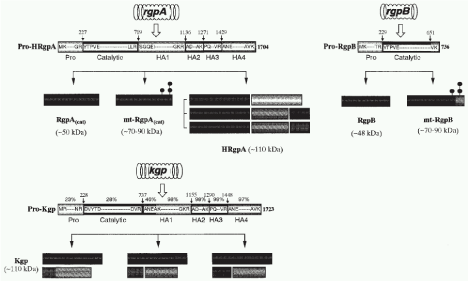
Figure 1: Isoform construction by proteolytic processing and assembly of the translated products of rgpA, agpB and kgp genes (HG66). Open arrows denote
translation of gingipain genes to their proforms. Pro, profragment; catalytic domain; HA1, HA2, HA3, HA314, HA4, heaogglutinin/adhesion subdomains. Intial and
endo amino acids of domains are shown. Small numbered arrows denote the cleavage site amino acid numbers and large arrows denote proteolytic processing
and assembly of the mature engyme complex, respectively. The domains with high homology are shown in the same pattern and percentage above Pro-Kgp
columns denotes homology percentage of the Pro-Kgp domains for the corresponding Pro-HRgpA domains. Dots attached to mt-RgpA(cat) and mt-RgpB denote
polysaccaharide chains. Molecula weights of isoforms are shown in parenthesers (Modified from potempa et al. [11]).
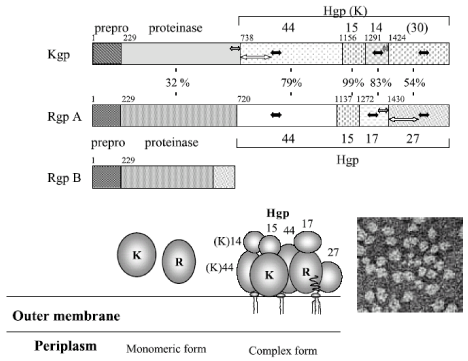
Figure 2: Schematic representation of the full length translation products from rgp and kgp genes and their structures. The numbers between RgpA and Kgp shows
the identical percentages in the amino acids of each domain. The solid, striped, and open arrows represent the well-conserved regions. The lower right panel shows
the electron micrograph of the purified membrane-associated gingipain complex.
Role of gingipains in host tissue damage and disease progression
The Arg- and Lys-proteinases have been either exposed at the surface (in the outer membrane) of the bacterium where they are able to come into contact with host cells and tissues or within the periplasmic space capable of being transported to the cell surface, and in outer membrane vesicles, which are sloughed from the outer membrane during growth [6,9] (Figure 3).
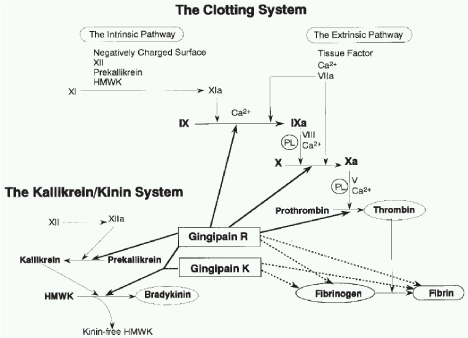
Figure 3: Effect of Gingipain R and K on clotting system of host.
Effect of Gingipain R and K on clotting system of host
Effect of Kgp fibrinogen lytic, leads to bleeding tendency during periodontitis. HrgpA potent thrombin producer, thrombin is not only invovled with blood clotting but also invoved with alveolar bone loss. Similarly Rgps increase Bradykinine production, which may lead to pain and alveolar bone loss. The gingipains are themselves, potent non-fimbrial adhesins avidly binding several extracellular matrix proteins such as fibrinogen, fibronectin, laminin, and collagen type V [14]. They also apparently mediate a tight adherence to epithelial cells and gingival fibroblasts with Kgp being implicated as providing most of the binding. In gingival microvasculature, vascular endothelial thrombomodulin easily and pertinently degrade by Rgp B than RgpA and Kgp.
Gingipains exert a sequential action on which Rgps converts oxyhemoglobin to methemoglobin, which render the hemoglobin more susceptible to degradation by Kgp [15]. The occurrence of gingipains in large complexes is a very cleaver design to facilitate hemoglobin degradation and the capture of the released heme is accomplished with high affinity by hemagglutinin-adhesin-2. Gingipains may function as hemophore-like proteins; shuttling captured heme to a Hemoglobin Receptor (HmuR) in the outer membrane.
Gingipains degrades monocytes CD14, a major receptor for bacterial LPS, rendering monocytes hyporesponsive to bacterial LPS. In densely populated biofilm, gingipains as well as proteases released by other periodontopathogens can proteolytically inactivate cationic antimicrobial peptides to enable the survival of other bacterial species which are highly sensitive to them [16]. Degradation of cationic antimicrobial peptides also inactivates cationic antimicrobial peptides’ ability to neutralize LPSs, which may lead to exacerbated, sustained production of proinflammatory cytokines [17].
P. gingivalis is resistant to killing by the human complement system. In a large part, this resistance is dependent on proteolytic activity of gingipains degrading different components of complement [18]. CXCL8 is an important signaling chemokine which is secreted in copious amounts by monocytes in response to infection and it serves to recruit neutrophils to the site of infection along a chemotactic gradient. Gingipains from Porphyromonasgingivalis play a significant role in induction and regulation of CXCL8 in THP-1 cells. Monocytes and neutrophils are sentinel cells of innate immunity and are found in abundance during periodontal infection [19]. THP-1 cells have been widely accepted and used as a surrogate for primary monocytes in biomedical research [20].
In addition, gingipains also contribute to proteolysis independent protection of P. gingivalis against complement-mediated lysis. This is achieved through the capture of the human complement inhibitor C4b-binding protein, thus, hindering deposition of the membrane attack complex on the P. gingivalis surface [21].
Gingipains efficiently degrade several extracellular matrix proteins in vitro gingipains can accomplish a lot more harm indirectly by disturbing the protease-protease inhibitor balance. In the case of human gingival fibroblasts, it was shown that matrix metalloprotease-1 expression was stimulated by Rgp activity [22]. Latent matrix metalloproteases can be directly activated by gingipains.
Conclusion
Natural or synthetic inhibitors of gingipains will lower the risk of periodontitis as well as peri-implantitis. The fimA type Ib genotype of P. gingivalis was found to play a critical role in the destruction of peri-implant tissue, suggesting that it may be a distinct risk factor for peri-implantitis [23]. Data suggest that immunization with RgpA stimulates the production of hemagglutinin domain-specific antibodies, which contribute to the prevention of P. gingivalismediated periodontal disease [24]. IgG and sIgA could be generated by immunization with rgpA DNA vaccine, which could significantly slow down bone loss in the experimental peri-implantitis canine model [25]. Vaccines, Gingipains inhibitors like chlorhexidine, Antibiotics like Tetracyclines are included in recent emerging treatment protocols against gingipains, but further human studies are necessary in this specific field as an adjunctive therapy in ameliorating chronic and aggressive periodontitis as well as peri-mucositis and peri-implantitis.
References
- Kamma JJ, Nakou M, Manti FA. Predominant micro- flora of severe, moderate and minimal periodontal lesions in young adults with rapidly progressive perio- dontitis. J Periodontal Res. 1995; 30: 66-72.
- Oliver RC, Brown LJ, Lo¨ e H. Periodontal diseases in the United States population. J Periodontol. 1998; 69: 269-278.
- Gibson FC 3rd, Savelli J, Van Dyke TE, Genco CA. Gingipain-Specific IgG in the sera of patients with periodontal disease is necessary for opsonophagocytosis of porphyromonas gingivalis. J Periodontol. 2005; 76: 1629-1636.
- Nakagawa T, Sims T, Fan Q, Potempa J, Travis J, Houston L, et al. Functional char- acteristics of antibodies induced by Arg-gingipain (HRgpA) and Lys-gingipain (Kgp) from porphyromonas gingivalis. Oral Microbiol Immunol. 2001; 16: 202-211.
- Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thøgersen IB, Enghild JJ, et al. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but in- dividual genes of Porphyromonas gingivalis. J Biol Chem. 1998; 273: 21648-21657.
- Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonasgingivalis. Periodontol 2000. 1999; 28: 168-238.
- Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J BiolChem. 1999; 274: 17955-17960.
- Ryan ME, Golub LM. Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy. Periodontol 2000. 2000; 24: 226-238.
- Imamura T. The Role of Gingipains in pathogenesis of periodontal diseases. J Periodontol. 2003; 74: 111-118.
- Honibald EN, Mathew S, Padmanaban IJ, Sundaram E, Ramamoorthy RD. Perioceutics: Matrix metalloproteinase inhibitors as an adjunctive therapy for inflammatory periodontal disease. J Pharm Bioallied Sci. 2012; 4: S417-S421.
- Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonasgingivalis. construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999; 274: 17955-17960.
- Lepine G, Progulske-Fox A. Molecular biology. In: Shah HN, Mayrand D, Genco RJ editor. Biology of the species porphyromonasgingivalis. Boca Raton: FL CRC Press; 1993. 293-319.
- Duncan MJ, Emory SA, Almira EC. Porphyromonasgingivalis genes isolated by screening for epithelial cell attachment. Infect Immun. 1996; 64: 3624-3631.
- Pathirana RD, O'Brien-Simpson NM, Veith PD, Riley PF, Reynolds EC. Characterization of proteinase-adhesin complexes of Porphyromonasgingivalis. Microbiology. 2006; 152: 2381-2394.
- Smalley JW, Birss AJ, Szmigielski B, Potempa J. Sequential action of R- and K-specific gingipains of Porphyromonasgingivalis in the generation of the haem-containing pigment from oxyhaemoglobin. Arch BiochemBiophys. 2007; 465: 44-49.
- Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, et al. Interpain A, a cysteine proteinase from prevotellaintermedia, inhibits complement by degrading complement factor C3. PLOS Pathog. 2009; 5: e1000316.
- Pandit N, Changela R, Bali D, Tikoo P, Gugnani S. Porphyromonas gingivalis : Its virulence and vaccine. J Int Clin Dent Res Organ. 2015; 7: 51-58.
- Potempa M, Potempa J, Okroj M, Popadiak K, Eick S, Nguyen KA, et al. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonasgingivalis. J Immunol. 2008; 181: 5537-5544.
- Jayaprakash K, Khalaf H, Bengtsson T. Gingipains from Porphyromonas gingivalis play a significant role in induction and regulation of CXCL8 in THP-1 cells. BMC Microbiol. 2014; 14: 193.
- Grenier D, Imbeault S, Plamondon P, Grenier G, Nakayama K, Mayrand D. Role of gingipains in growth of porphyromonas gingivalis in the presence of human serum albumin; Infect Immun. 2001; 69: 5166–5172.
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000. 2010; 54: 15-44.
- Matsushita K, Imamura T, Tomikawa M, Tancharoen S, Tatsuyama S, Maruyama I. DX-9065a inhibits proinflammatory events induced by gingipains and factor Xa. J Periodontal Res. 2006; 41: 148-156.
- Kim SG, Hong JY, Shin SI, Moon JH, Lee JY, Herr Y. Prevalence of Porphyromonas gingivalis fimA genotypes in the peri-implant sulcus of Koreans assessed using a new primer. J Periodontal Implant Sci. 2016; 46: 35-45.
- Gibson FC 3rd, Genco CA.. Prevention of Porphyromonas gingivalis-Induced oral bone loss following immunization with gingipain R1. Infect Immun. 2001; 69: 7959-7963.
- Fan X, Wang Z, Ji P, Bian Y, Lan J. rgpA DNA vaccine induces antibody response and prevents alveolar bone loss in experimental peri-implantitis. J Periodontol. 2013; 84: 850-856.