
Research Article
J Dent & Oral Disord. 2019; 5(2): 1116.
The Effectiveness of 980nm Laser Therapy on Socket Preservation: A Preliminary Split Mouth Randomized Controlled Clinical Study
Abdel Mageed H1,2†, Hanna R2†* and Benedicenti S2
¹Laser Dental Center, Building 40A Baghdad Street, El Korba, Heliopolis, Egypt
²Laser Therapy Center, Department of Surgical and Diagnostic Sciences, University of Genoa, Italy †Joined first author
*Corresponding author: Hanna R, Department of Surgical and Diagnostic Sciences, University of Genoa, Genoa, Italy
Received: August 05, 2019; Accepted: September 12, 2019; Published: September 19, 2019
Abstract
Background: The area of interest of laser beam profile and its clinical application have examined previously. However, utilization 980nm diode laser treatment in this concept remains unevaluated, in order to verify its potential in socket preservation. Our study’s objectives were to examine 980nm beam profile zones, high-level laser therapy and low-level laser therapy, in enhancing the haemostasis and accelerating bone healing in extraction socket respectively.
Methods: Ten recruited orthodontic patients required bilateral lower first premolars teeth extraction. Twenty sockets randomized into Laser Group (LG) and Control Group (CG). Two laser treatment protocols utilized as follows: contact stage (2 Watts, Continuous Wave (CW), 300 microns tip, 14 seconds exposure time) and non-contact stage (3 Watts, CW, 300 microns tip, 40 seconds exposure time). Cone Beam Computer Tomography (CBCT) immediately and 4 months postoperatively utilised to measure bone height, width, density, and a neoformed bone in extraction sockets.
Results: Four months CBCT values of bone density and newly formed bone were higher of mean values 498.61 mm ± 142.5 and 1.63 mm ± 0.22 respectively, and statistically significant differences P=0.04 and P=0.17 respectively, in LG compared to CG.
Conclusion: Our data, for the first time, prove the positive effects of 980nm laser beam profile zones in socket preservation.
Keywords: Bone regeneration; Bone repair; High-Level Laser Therapy (HLLT); Low-Level Laser Therapy (LLLT); Photobiomodulation; Tooth socket preservation
Introduction
Physiological bone loss is one of the unavoidable complications, which is associated with tooth extraction. Most of the alveolar bone resorption occurs during the first 3 to 6 months after tooth extraction [1-3]. Therefore, the socket preservation has become the gold standard in recent years among clinicians. Several in-vivo studies, clinical case series and systematic reviews published in order, to establish the rationale in using socket preservation as a therapeutic therapy [4-7]. However, some of these techniques can incur some challenges. Photobiomodulation (PBM) therapy has been utilised to enhance bone healing for over the last 3 decades. Despite many studies examined the positive effects of PBM and HLLT on early bone healing [8-11], it still remains a fact that insignificant difference observed in bone regeneration between the control and irradiated groups [12].
When the extraction socket irradiated with 980nm, the photonic energy of its first zone beam profile (HLLT) absorbed by the Haemoglobin (Hb), which subsequently transformed into a thermal energy (Photothermolysis), in order to achieve photocoagulation [13]. The protein in the blood denatures at 60 to 65 degree Celsius (°C); due to the photothermolysis effect [14]. The bone of the extraction socket walls can gain the Low-Level Laser Therapy (LLLT) effect in the last zone of the beam profile [15,16]. This enhances the neovascularization, increases collagen synthesis, and promotes newly formed bone. This is due to the 980nm bio-stimulatory effect on gene expression up- regulation, growth factors increase and cell proliferation and differentiation [17,18]. The 980nm mechanism of action is different than any other wavelengths in the IR region, as the main chromophore for PBM is water [19].
Many studies showed the effectiveness of 980nm PBMT on tissue regeneration, as an adjunctive therapy to the non-surgical periodontal treatment [20,21]. Ohshiro et al.1996, stated that the effects of laser energy on the tissue varies, which mostly depending on the power density or on the photonic density of the incident beam on the target tissue [18]. This led to a progression of the photodestructive and photoactivation sides of the laser beam profile. The photoreactions are temperature specific. However, the range of changes in the temperature depends mostly on the laser wavelength and target tissue phenotype. Ohshiro et al.1996 identified two zones of photoreaction in the laser beam profile; first zone, the photodestruction associated with the laser surgery for which called HLLT, while the second one, the photobioactivation associated with laser therapy, subablative threshold, for which is LLLT [18]. The school of thought among various orthodontic researchers is prevention modality prior to orthodontic tooth movement to avert the possibility of bone resorption and dehiscence [22].
Therefore, our study, for the first time, examined the effect 980nm laser beam profile concept on extraction socket preservation. The objectives of the study were to investigate, whether using 980nm could enhance the hemostasis process, through its coagulation phenomena, and accelerate the bone healing (remodeling sage) through its LLLT effect.
Material and Methods
Inclusive and inclusive criteria
Ten healthy and non-smoking (6 females and 4 males) patients at age range between 17-30 year old, enrolled in a comparative split mouth randomised controlled clinical trial (Figure 1). All the recruited patients required extraction of bilateral lower first premolar teeth for orthodontic purposes and presented with a good oral hygiene with plaque index of “0” value, according to Silness and Loe classification [23]. The treatment side of each patient was allocated using a Google random number generator, leading to a right or left treatment side only (laser group). The un-selected site received a sham laser therapy (control group). The total data were 20 extraction sockets without socket preservations. The study was double blinded (patient and investigator). The exclusive criteria were opposite to the above, including pregnant women. This study conformed to CONSORT guidelines. All the subjects gave their informed consent for inclusion, before they participated in the study, which was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of October 6 University/Faculty of Dentistry/Egypt. The project identification code is 109, which was issued on the 02/05/2017.
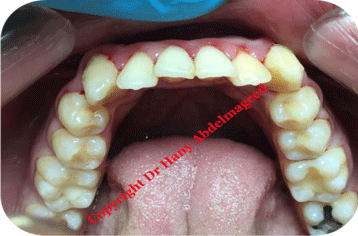
Figure 1: Shows pre-operative view of the lower arch, presenting bilateral
lower first premolar.
Treatment protocol
The periotome utilized to achieve atraumatic extraction [24]. A single operator performed the clinical examinations and carried out the extraction and the laser treatments. All data recorded by a senior dental nurse and then stored on Excel software programme. Followup appointment was scheduled 4 months post-treatment.
Laser wavelength specifications and treatment protocol
The 980nm diode laser (Doctor Smile Wiser/LA3D0 001.3) was employed in this study. The device is LAMBDA SpA Technologies (Brendola, Vicenza. ITALY). The protocol of the laser treatment stages was based on the investigator previous clinical practice protocol with good results.
The stages of the laser treatments are as follows:
1. The first treatment protocol (contact stage) (Figures 2 & 3): when the blood filled the extraction socket (without oozing) immediately after extraction, uninitiated 300 microns tip utilized at a power output of 2 Watts (W) in a continuous emission mode (CW) with an average power density 2829 W/cm2. The total energy was 28 Joules (J), with an energy density of 330.6 J/cm2 at speed movement of 2 mm/second for 14 seconds (Table 1). The setting time here was crucial to avoid any possible high temperature, as prolonged timing inside the socket can result in temperature increase and prolonged coagulation time. Therefore, timing is the key factor here [11]. The fibre tip movement started from the bottom of the socket upward in a circular movement, until it reached the demand of the blood viscosity and the clotting effect. The laser device was on until the complete removal of the tip from the socket, to ensure in-situ clot. The acceleration mechanism of the clotting formation observed clinically.
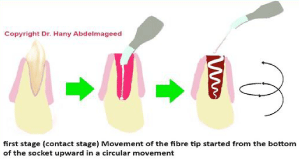
Figure 2: Illustrates the steps’ sequence of the first stage of the laser
treatment (fibre tip movement and direction).
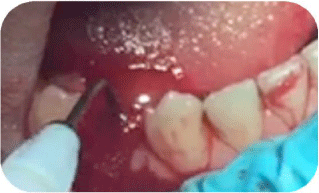
Figure 3: Shows a peri-operative view of the first stage of the laser therapy
where the tip of the 980nm fibre was inside the socket of the lower first
premolar.
Emission mode
Continuous emission mode (CW)
Power
2 Watts
Power Density
2829 W/cm2
Spot diameter at tissue
0.0300 cm
Spot area at tissue
0.0007 cm2
Diameter of the tip
300μm (microsecond)
Speed of movement
2 mm/sec (seond)
Energy Density with movement
330.6 J/cm2
Total energy
28 J (Joul)
Treatment Time
14 seconds
Frequency of treatments
Once immediately after the extraction
Total number of treatments
Once
Technique used in the treatment
Contact mode
Treated area (Location)
Inside the extracted socket in a circular movement.
Table 1: The laser treatment parameters for first stage (contact stage).
2. The second treatment protocol (non-contact stage) (Figures 4, 5a & 5b): the aim of this stage was to form the hard-clotting band, in order to stabilize the coagulated blood. This achieved with uninitiated 300 microns tip at 3 W in a CW, when the tip was 1mm away from the target tissue in a scanning motion at a speed movement of 2 mm/second for 40 seconds. The average power density was 678 W/cm2. The total energy was 120 J whilst the energy density with movement was 393.8 J/cm2 with a spot area at tissue of 0.0044 cm2 (Table 2). Figure 6 shows the control and laser sites immediately posttreatment.
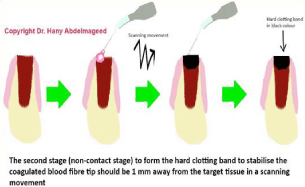
Figure 4: Illustrates the steps’ sequence of the second stage of the laser
treatment (fibre tip movement).
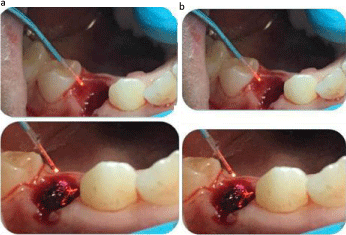
Figure 5a: Shows the second stage of the laser treatment when the tip of the
980nm fibre, forming the hard-clotting band; Figure 5b: Shows the second
stage of the laser treatment when the tip of the 980nm fibre completed the
hard clotting band formation.
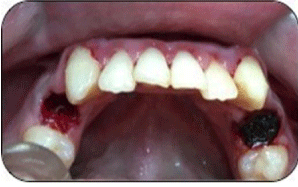
Figure 6: Shows a comparison of the clotting status between the control and
the laser (right socket) treated sockets, immediately post-treatment.
Emission mode
Continuous emission mode (CW)
Power
3 Watts
Power Density
678 W/cm2
Spot diameter at tissue
0.0751 cm
Spot area at tissue
0.0044 cm2
Speed of movement
1 mm/sec (second)
Energy Density with movement
393.8 J/cm2
Total energy
120 J
Treatment Time
40 second
Frequency of treatments
Once immediately after the extraction
Total number of treatments
Once
Technique used in the treatment
Non-contact
Diameter of the tip
300μm
Treated area (Location)
1mm away from the occlusal surface of the extracted socket in a scanning movement.
Table 2: The laser treatment parameters for second stage (Non-contact).
Cone Beam Computed Tomography (CBCT) specifications and protocol
All the patients had CBCT of the mandible immediately after extraction and 4 months postoperatively for both groups, in order to evaluate the late stage of bone healing (remodeling phase), bone density, and bone width and height. Authors were keen to demonstrate the effect of LLLT on newly matured bone formation (remodeling stage). Therefore, they setup this CBCT protocol at 4 months postoperatively. A specialist oral and maxilla-facial radiologist carried out the CBCT for all the recruited patients and was blinded regarding the laser treatment sites throughout the study and data analysis.
Two different CBCT machines utilised; the Planmeca ProMax 3D Mid and the Vatech Green CT.
The specifications of these two machines are as follows:
Planmeca ProMax 3D Mid: This is a genuine all-in-one CBCT (Cone Beam Computed Tomography) unit, including 3D imaging, 3D photo, digital 2D panoramic and cephalometry. All these are of the same unit with a versatile volume sizes for teeth Ø40 x 50 mm (Ø34 x 42 mm), Ø40 x 70 mm (Ø34 x 60 mm), Ø70 x 50 mm (Ø60 x 42 mm), Ø70 x 70 mm (Ø60 x 60 mm), Ø90 x 50 mm (Ø75 x 42 mm), Ø90 x 90 mm (Ø75 x75 mm) and for Jaw Ø160 x 50 mm (Ø160 x 50 mm), Ø160 x 90 mm (Ø160 x 90 mm) and for Face Ø160 x 160 mm (Ø160 x 160 mm) with a Voxel size, isotropic 100 μm, 150 μm, 200 μm, 400 μm, 600 μm, X-ray beam is Cone, Anode voltage 54–90 kV, Anode current is 1–14 mA, Focal spot is 0.5 mm and fixed anode, the Image detector is Flat panel, Gray scale of 15 bit, Detector resolution 127 μm, Image acquisition 210/360 degree rotation, Total scan time 18–26 seconds and pulsed X- ray, Reconstruction time 15 seconds at minimum, 3D reconstruction server Proprietary Feldkamp type back projection reconstruction algorithm with Improved Artefact Removal (IAR) for high contrast object compensation.
Vatech Green CT: This is a CBCT (Cone Beam Computed Tomography) unit, including CBCT and panorama with a Computed Tomography Field of View size (CT-FOV SIZE) 15x15 cm: Multi [5x5/8x5/8x8/12x9/15x15 cm] and VOXEL SIZE 5x5 cm:0.08 mm/ 0.2 mm, 8x8, 12x9 cm : 0.25 mm/0.3 mm, 15x15 cm : 0.25 mm/0.3 mm, SCAN TIME for Panorama : 10.1 sec and SCAN TIME for CBCT: 5.9 sec, 9 sec, GRAY SCALE of 14 bit, TUBE VOLTAGE/ CURRENT 50-100 kVp (1 kV step)/4-16 mA (0.1 mA step) Vatech Green CT is an ultra-low X-ray dose.
Planmeca Romexis® software release 4.6.0.R utilised
Bucco-lingual dimension with a slice thickness of 0.9 mm, and length of 30 mm employed for all the cases. In immediately and 4 months after extraction CBCT, the height measurement was recorded from the top of the socket (crestal bone) till the end of the cancellous bone while the width recorded from the buccal cortical plate to the lingual cortical plate (Figure 7). Only the bone density measurements were undertaken at the 4 months post extraction CBCT at the region of a newly formed woven bone at cortical bone (Figure 8). The CBCT tool to detect mineral, which is the densest component in bone tissue, and provide a Hounsfield units, as the measures.
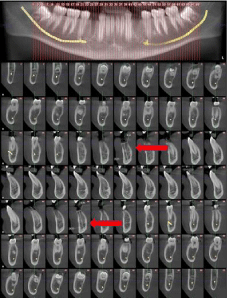
Figure 7: Illustrates the bone height, immediately after treatment. the height
measurement was done from the top of the socket at the alveolar crest
bone till the end of the cancellous bone, whilst the width measurement was
measured from the buccal cortical plate to the lingual cortical plate. The red
arrows represent the reference points of measurements.
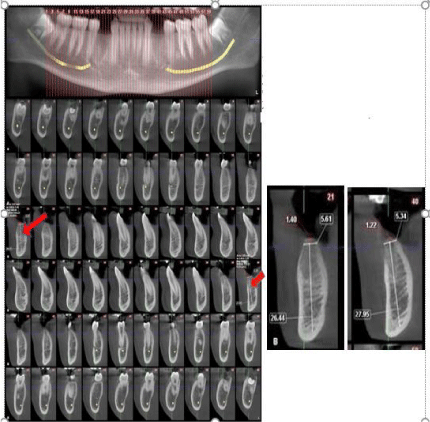
Figure 8: Shows the CBCT images of the bone height, bone density and
newly formed bone four months postoperatively.
The CBCT measurements of the bone height was calculated from the top of
cortical bone till the end of the cancellous bone, whilst the width measurement
was calculated at reference point from the buccal cortical plate to the lingual
cortical plate, which were the same reference points that were used in the
immediately after extraction CBCT. Only the bone density measurements
were noted at four months postoperatively.
Statistical analysis
The values were presented as mean and Standard Deviation (SD). The data were explored for normality, using Kolmogorov-Smirnov test of normality. The results of Kolmogorov-Smirnov test indicated that the height, width, density and newly formed bone data were normally distributed (parametric data). So, independent t test was used to compare between the groups, while the paired t test was used to examine the effect of time within the same group. The data that are related to percent change in bone and height were not normally distributed (non-parametric data). So, Mann Whitney U test was used to compare between groups. The significance level (Statistical power) was set at p=0.05. Statistical analysis was performed with SPSS 19.0 (Statistical Package for Scientific Studies, SPSS, Inc.) for Windows.
Results
The results showed a higher mean value of bone density (498.61 ± 142.5) recorded 4 months post-operatively in the laser group (Figures 7 & 8). Independent t test revealed that this difference was statistically significant (P=0.04) (Table 3 and Figure 9). With reference to the newly matured formed bone at 4 months post treatment, the CBCT showed a higher mean value (1.63 ± 0.22) recorded in the laser group (Figures 6, 7). Independent t test revealed that this difference was statistically significant (P=0.17) (Table 4, Figure 10). However, our study’s results did not show any statistically significant increase in the width and height of the extraction sockets on CBCT in the laser group 4 months post-operatively in comparison to the control group at P=0.98 and P=0.719 respectively (Tables 5,6,7).
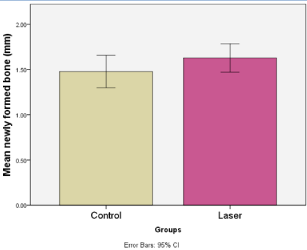
Figure 9: Shows the mean values of CBCT newly formed bone in the control
and laser groups.
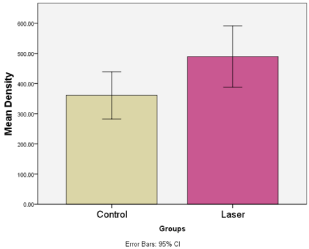
Figure 10: Shows the mean values of CBCT bone density in the control and
laser groups.
Group Statistics
Difference
Groups
Mean of the ounsfield unit
Std. Dev
Std. Error
Mean
Std. Error
CI Lower
CI Upper
t
P
Density
Control
360.81
109.5
34.63
-128.8
56.83
-248.77
-8.83
-2.27
.04*
Laser
489.61
142.5
45.06
Std. Dev: Standard Deviation, Std. Error: Standard Error, 95% Confidence Interval of the Difference= 95%, Lowe: Lower Jaw, Upper: Upper Jaw, C.I: Significance level p<0.05, *significant
Table 3: Comparison of CBCT bone density values between control and laser groups (Independent t test) 4 months post- operatively shows a statistical significant differences in the laser group.
Group Statistics
Difference
Groups
Mean of the ounsfield unit
Std. Dev
Std. Error
Mean
Std. Error
CI Lower
CI Upper
t
P
Newly Formed bone
Control
1.48
0.25
0.08
-0.15
0.11
-0.37
0.07
-1.43
.17*
Laser
1.63
0.22
0.07
Std. Dev: Standard Deviation, Std. Error: Standard Error, 95% Confidence Interval of the Difference: 95% C.I., Significance level p<0.05, *significant.
Table 4: Comparison of CBCT newly formed bone (mm) values between control and laser groups 4 months post-operatively (Independent t test) shows a statistical significance differences in the laser group.
Groups
Groups
Difference
Mean
Std. Dev
Std. Error
Mean
Std. Dev
Std. Error
CI lower
CI upper
t
p
Control
Immediately
7.71
0.75
0.24
1.63
0.89
0.28
0.99
2.26
5.77
.00*
After 4 months
6.09
0.9
0.28
Laser
Immediately
7.87
0.77
0.24
1.69
0.72
0.23
1.18
2.21
7.44
.00*
After 4 months
6.18
0.9
0.28
Std. Dev: Standard Deviation, Std Error: Standard Error, 95% Confidence Interval of the Difference: C.I., Lower: Lower Jaw, Upper: Upper Jaw, Significance level p<0.05 *significant
Table 5: Comparison of CBCT bone width (mm) values (paired t test) between the control and laser group, 4 months post treatment: it shows a statistically significance differences, immediately and 4 months post-treatment.
Group Statistics
Difference
Groups
Mean
Std. Dev
Std. Error
Mean
Std. Error
CI
CI
t
P
Lower
Upper
Immediately
Control
7.71
0.75
0.24
after extraction
Laser
7.87
0.77
0.24
-0.16
0.34
-0.87
0.56
-0.47
.65ns
After 4
Control
6.09
0.9
0.28
months
Laser
6.18
0.9
0.28
-0.09
0.4
-0.93
0.75
-0.23
.82ns
Std. Dev: Standard Deviation, Std. Error: Standard Error, t: Independent t test, Lower: Lower Jaw, upper: Upper Jaw. 95% Confidence Interval of the Difference: C.I. Significance level p<0.05, ns: non-significant
Table 6: Comparison of CBCT bone width (mm) between control and laser group (Independent t test), showing no statistical significance differences immediately and 4 months post-treatment.
Group Statistics
Difference
Groups
Mean
Std. Dev
Std. Error
Mean
Std. Error
CI
CI
P
Lower
Upper
Control
0.97
3.82
1.21
Laser
0.59
4.27
1.35
0.38
1.81
-3.43
4.19
.84ns
Std. Dev: Standard Deviation, Std Error: Standard Error, 95% Confidence Interval of the Difference= C.I., Significance level p<0.05, ns=non-significant.
Table 7: Comparison of CBCT bone height (%) between the control and laser groups, showing no statistical significance differences (Mann Whitney U test).
Discussion
The rationale for extraction socket preservation is to reduce the loss of alveolar bone to acceptable levels [6]. Bone regeneration in the field of dentistry and oral and maxillofacial surgery has become the challenge among most clinicians. Phototherapy can add value in accelerating the bone healing. Santinoni et al. 2017 systematic review concluded that LLLT effects on bony density increase in surgical defects was recorded. However, lack of quality studies to support the effect of 980nm towards bone repair was related to heterogeneity of the laser parameter protocols [25]. Moreover, several studies investigated the effect of LLLT on the initial stage of bone regeneration after tooth extraction (30-45 days) but complete osteogenesis was unconfirmed [26,27]. Garcia et al. 2014 [26] study showed a newly formed immature bone occupied more than two-thirds of the surgical wound after 30 days in the laser group. The centre of the bone defect was filled with connective tissue, which was evaluated via panoramic view (early bone formation). Furthermore, Romão et al. 2015 human study [27] evaluated the effect of 808 nm on human alveolar bone, using micro-computed tomography. An increase in the bone density (P < 0.0001) in the lased sockets noted 40 days after the extraction. This represents initial stages of osteogenesis, but by no means it signifies the completion of the bone regeneration process [27], while Kucerova et al. 2000, reported statistically insignificant changes in the bone density by comparing data collected immediately and 6 months after extraction. As there was no measurement of the bone healing in the interval period between these two-time events, it was inconclusive to report whether laser therapy can induce similar bone density prior to this [28]. Beresescu et al. 2015 histological study suggested that in 6 months, there was an evidence of a newly bone formation in the defects treated with LLLT of 618nm, which indicates the possibility of more rapid wound closure and subsequent healing [29]. In the context of the effect of LLLT in improving the periodontal parameters, Pejcic et al. 2010 demonstrated the beneficial effect of LLLT on patients with periodontal diseases, as significant improvements in the periodontal parameters after 6 months noted [30]. Therefore, our work, for the first time, has shed a light on this inconclusive outcome. A statistically significant increase in bone density and newly formed bone observed in the laser group in comparison to the control after 4 months (remodeling stage) (Figure 3) can bridge the current gap in literature.
Aoki et al. 2008 study showed the effect HLLT on the ablation of the periodontally diseased tissues, which was partly, had a simultaneous PBM effect on the surrounding tissues. In periodontal pocket therapy, laser can assist not only in ablating the diseased tissues but also in bone healing [31]. This modality can be utilized more effectively, as an adjunctive tool to the current mechanical therapy. Furthermore, Sella et al. 2015 [32] utilised diode 808 nm in a randomised control trail (in- vivo animal) study, to evaluate the effect of PBM on newly formed bone over period of 8, 13, and 18 days. The results showed a statistically significance of P ‹ 0.001 of a newly formed bone at 18 days in comparison to the control group (early stage of bone healing). However, there was no record of late stage of bone healing, in order to demonstrate the complete osteogenesis [32]. Surprisingly, Mohammed et al. 2015 in-vivo animal study [33] utilised 632.8 nm and 905 nm to induce biomodulatory effect on bone regeneration. The infrared laser showed an evidence of more bone repair. However, there was no difference between the two wavelengths after 45 days post-treatment [33]. It is important to mention that our work coincided with Deppe et al. 2001 animal study’s results showed that the re-established bone-to-implant contact was significantly greater in the irradiated group with carbon dioxide laser than in the conventionally treated group, which assessed radiographically 6 months after extraction. It is important to mention that the former study utilised 2.5 W in CW, which had no thermal effect on the implant surface and induced bone healing [34]. Mirdan et al. 2012 animal study observed extraction socket coagulation when 980nm irradiation utilised at power output setting of 0.86 W [35]. However, in our study we utilised 2W, taking in account the scattering phenomena, the wavelength, and the distance between the laser beam and the target.
Vuylsteke et al. 2009 in-vivo animal study showed at range of 940 nm - 980nm wavelengths when the scattering and the absorption coefficients were approximately 0.6 - 0.64 mm-1 and 0.25 - 0.28 mm-1 in blood respectively [36]. These figures confirmed that the clotting process in the current study achieved due to the absorption phenomena. In addition, the scattering factor limited the laser photothermal effect without to be conserved on the clot formation only, leaving the normal surrounding bone unaffected but instead its healing may be stimulated by the remaining scattered light from the incident laser [20]. Furthermore, Ohshiro et al. 1996 demonstrated the effect of the surgical laser on tissue with the zones of thermally dependent reactions from high temperature at certain impact point to a little heat in the deeper layers [18]. This justifies our chosen power output, which stimulated a newly formed bone without any heat adverse effects. Moreover, the photothermal effect of 980nm at 2W accelerated the clotting process without any impact on the function of aggregated platelets. This confirms that first stage clotting temperature maintained ‹ 42°C, which is safe [37]. This validates that the heat production of HLLT was not readily transmitted to the alveolar bone of the extraction socket. Moreover, Eriksson et al. 1984 examined the effect of defined temperature rise on bone healing in animal experimental study, which concluded that no adverse effect if the temperature maintained ‹ 44°C for 1 minute [38]. Leja et al. 2013 study investigated the initiated and uninitiated tips and associated thermal effects on tissue regeneration. The findings related to the 980nm absorption affinity to water, which was approximately 35%, in comparison to 810nm and 1064nm, which was 3% and 15% respectively. The 980nm surgical incision is more optically than thermally, as its photonic energy is absorbed versus the thermal effect generated by other wavelengths [39]. Continuous movement of the laser tip can dissipate some of the heat. Therefore, spiral movement at speed of 2mm/second implemented in our study.
Romanos et al. 2019 assessed the photothermal effect of 980nm defocused initiated versus uninitiated tip, in infra-bony defect of dental implant for decontamination purposes. The results showed that blue-initiated tip had the highest temperature increase (22.4°C), followed by cork (18.8°C) and uninitiated tip (17.3°C). The critical threshold at the coronal portion of 980 nm laser tip reached in 11.5, 8.79, and 6.46 seconds for the blue paper, cork, and uninitiated tips respectively [40]. Hudson et al. 2013 study [41] showed that 980nm penetration depth was approximately 2.2 cm, which sufficient to stimulate bone healing. Furthermore, Wang et al. 1995 [42] demonstrated that both the absorption and the scattering phenomena were significantly less, as the wavelength gets longer. Subsequently, they have a deeper penetration depth. Our work exhibited that 980nm HLLT penetration depth was satisfactory to stimulate bone.
It is impossible to establish a comparison between the present study and any previous reports, due to the lack of similar reported experimental studies. So, we can assume that the bone healing was due to a combined effect of 980nm HLLT and LLLT and late osteogenesis shown after 4 months. Finally, this concept can be considered as a preventive modality prior to orthodontic tooth movement, to avert the possibility of bone resorption and dehiscence [22]. This is the current school of thought among the orthodontic researchers.
Conclusion and Future Perspective
Based on the results obtained and according to the methodology employed in the current study, 980nm beam profile zones has proven to accelerate the clotting mechanism, through the photocoagulation phenomenon, and increase the bone density and the newly formed bone in an extracted socket, through the concept of HLLT inducing PBM effect. This is a useful tool in socket preservation and bony defects related to various metabolic disorders. Within the limitation of this study, The author suggest further comparative split mouth randomized controlled trial studies with large data, required to reinforce standarisation of our laser treatment protocol in socket preservation application. Ultimately, would contribute in conforming our laser treatment protocol for this novel approach in socket preservation.
Acknowledgement
The authors would like to express their gratitude to the Premier Laser Dental Center staff, at both Heliopolis and Sheikh Zayed sites, in Egypt for their great support in collecting and recording the data and patients’ recruitment process. Moreover, the authors would like to acknowledge Dr Amr Tuhami’s expertise and knowledge (Radiologist consultant specialized in computer guided surgery & CBCT) along with his colleague, Dr. Ahmed Seifeldin, in performing the CBCT and interpreting the images (Photon Dental Scan Centre in Egypt).
References
- Schropp L, Wenzel A , Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23: 313-323.
- Virdi M. Oral Health Care- Prosthodontics, Periodontology, Biology, Research and Systemic Conditions. 2012.
- Hansson S, Halldin A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. Journal of Dental Biomechanics. 2012; 3: 1-8.
- Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J. Periodontol. 1997; 68: 563-570.
- Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998; 69: 1044-1049.
- Hämmerle CH, Araujo MG, Simion M. Osteology Consensus G. Evidencebased knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012; 23: 80-82.
- Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012.
- Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 1998; 22: 97-102.
- Marques L, Holqado L, rancischone FL, Ximenez J, Okamoto R, Kinoshita A. New LLLT protocol to speed up the bone healing process histometric and immune histochemical analysis in rat calvarial bone defect. Lasers Med Sci. 2015; 30: 1225-1230.
- Kim K, Kim IS, Cho TH, Seo YK, Hwanq SJ. High intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J Tissue Eng Regen Med. 2015; 9: 943-951.
- Bouvet-Gerbettaz S, Merigo E, Rocca JP, Carle GF, Rochet N. Effects of lowlevel laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 2009; 41: 291-297.
- Zhang JZ, Zhang XX, Audette M. Photothermal model of selective photothermolysis with dynamically changing vaporization temperature. Laser Med Sci. 2011; 26: 633-640.
- Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomedicine and laser surgery. 2004; 22: 411-417.
- Ohshiro T, Fujino T. Laser applications in plastic and reconstructive surgery. The Keio Journal of Medicine. 1993: 42: 191-195.
- Convissar RA. Principles and Practice of Laser Dentistry 2nd edn. Elsevier Health Sciences. 2015.
- Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers in Surgery and Medicine. 1998: 22: 97-102.
- Weber JB, Pinheiro AL, Oliveira MG, Oliveira FA, Ramalho LM, Luciana Maria P, Lamarho, et al. Laser therapy improves healing of bone defects submitted to autologus bone graft. Photomedicine and laser surgery. 2006; 24: 38-44.
- Ohshiro T. The laser apple: a new graphic representation of medical laser applications. Laser Therapy. 1996; 8: 185-190.
- Wang Y, Huang Y, Wang Y, Lyu P, Hamblin M. Photobiomodulation of human adipose- derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta. 2017; 1861: 441–449.
- Kamma JJ, Vasdekis VG, Romanos GE. The Short-term Effect of Diode Laser (980nm) Treatment on Aggressive Periodontitis. Evaluation of Clinical and Microbiological Parameters. J Orala Laser Applications. 2006; 6: 111-121.
- Park JJ, Kang KL. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers in Medical Science. 2012; 27: 223-230.
- Dominguez A, Leon P, Aristizabal J. Effect of low-level laser therapy on local bone resorption during orthodontic treatment. A randomized controlled trial. Int J Odontostomat. 2016; 10: 483-490.
- Silness J, Loe H. Periodontal disease in pregnancy 2. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964; 22: 121- 135.
- Sharma SB, Vidya B, Alexander M, Deshmukh S. Periotome as an Aid to Atraumatic Extraction: A Comparative Double Blind Randomized Controlled Trial. Journal of Maxillofacial and Oral Surgery. 2015; 14: 611-615.
- Santinoni CD, Oliveira HF, Batista VE, Lemos CA, Verri FR. Influence of low level laser therapy on the healing of human bone maxillofacial defects: A systematic review. Journal of photochemistry and photobiology B, Biology. 2017; 169: 83.
- Garcia VG, Sahyon AS, Longo M, Fernandes LA , Gualberto Junio EC, Noronha Novaes VC, et al. Effect of LLLT on autogenous bone grafts in the repair of critical size defects in the calvaria of immunosuppressed rats. Journal of Cranio-Maxillo-Facial Surgery. 2014; 42: 1196-1202.
- Romão MM, Marques MM, Cortes AR, Horliana AC, Moreira MS, Lascala CA. Micro- computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: a pilot study. International Journal of Oral and Maxillofacial Surgery. 2015; 44: 1521-1528.
- Kucerová H, Dostálová T, Himmlová L, Bártová J, Mazánek J. Low-level laser therapy after molar extraction. J Clin Laser Med Surg. 2000; 18: 309-315.
- Beresescu G, Monea M, Porca B, Cocan A, Monea A. Effects of Low-Level Laser Therapy on Bone Regeneration of Intrabony Defects. Key Engineering Materials. 2015; 638: 151-154.
- Pejcic A, Kojovic D, Kesic L, Obradovic R, The effects of low-level laser irradiation on gingival inflammation, Photomed Laser Surg. 2010; 28: 69-74.
- Aoki A, Takasaki AA, Pourzarandian A, Mizutani K, Ruwanpura SM, Iwasaki K, et al. Photobiomodulation Laser Strategies in Periodontal Therapy. Lecture Notes in Electrical Engineering (LNEE). 2008; 12: 181-190.
- Sella VR, do Bomfim FR, Machado PC, da Silva Morsoleto MJ, Chohfi M, Plapler H. Effect of low-level laser therapy on bone repair: a randomized controlled experimental study. Lasers in Medical Science. 2015; 30: 1061- 1068.
- Mohammed A, Mostafa YM, Abdl-Azizi NT. Study of the Effect of Low Level Laser Therapy on Bone Repair in Rats. JMSCR. 2015; 3: 8275-8286.
- Deppe H, Horch HH, Henke J, Donath K Deppe H, Horch HH, Henke J, Donath K. Peri-implant care of ailing implants with the carbon dioxide laser. Int J Oral Maxillofac Implants. 2001; 16: 659-667.
- Mirdan BM. A 980nm Diode Laser Clot Formation of the Rabbit’s Dental Sockets after Teeth Extraction. Iraqi J Laser. 2012; 11: 37-42.
- Vuylsteke M, Van JD, Roelens J, De TB, Mordon S. Endovenous laser treatment: a morphological study in an animal model. Phlebology. 2009; 24: 166-175.
- White J. Effects of heat on platelet structure and function. Blood Journal. 1968; 32: 324-335.
- Eriksson RA, Albrektsson T. The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg. 1984; 42: 705-711.
- Leja C, Geminiani A, Caton J, Romanos GE. Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study. Lasers Med Sci. 2013; 28: 1435-1440.
- Romanos GE, Motwani SV, Montanaro NJ, Javed F, Delgado-Ruiz R. Photothermal Effects of Defocused Initiated Versus Noninitiated Diode Implant Irradiation. Photobiomodulation, Photomedicine, and Laser Surgery.
- Hudson DE, Hudson DO, Wininger JM, Richardson BD. Penetration of Laser Light at 808 and 980nm in Bovine Tissue Samples. Photomedicine and Laser Surgery. 2013; 31: 163-168.
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Subj. 2017; 1861: 441-449.