
Research Article
J Dent & Oral Disord. 2020; 6(1): 1125.
Heat Generation and Temperature Increase in the Root during Electromagnetic Apical Treatment
Tominaga T1*, Tada E1,2, Takahira K1,3 and Sugaya T2
¹Department of Tominaga Dental Clinic, Shimohonjo Akinokami Seto Naruto, Japan
²Department of Periodontology and Endodontology, Hokkaido University, Japan
³Department of Management and Information Science, Shikoku University, Japan
*Corresponding author: Toshihiko Tominaga, Department of Tominaga Dental Clinic, Shimohonjo Akinokami Seto Naruto, Japan
Received: February 03, 2020; Accepted: February 24, 2020; Published: March 02, 2020
Abstract
The temperature increase due to high-frequency energization reduces bacterial viability. However, the harmful effects of overheating of the periodontal tissues during a root canal procedure are a matter of concern. Therefore, we examined the heat generation and temperature increase in an active electrode on the intracanal and root surfaces by energization and to develop a novel endodontic treatment system using high-frequency current. K-file #10–40/.02 T was inserted into an egg white at several insertion depths. The current flow was energized with 510-kHz frequency at the maximum output of 15.5 W for 1.0 s, and coagulation was evaluated. K-file was inserted at 2.0 mm on the crown side from the apical foramen of a mandibular anterior tooth, followed by energizing ten times. The temperatures of the intracanal and root surfaces were measured at the portion by a thermocouple Type-K TC-K-F-0.1-WP (HAYASHI DENKO Corp., Tokyo, Japan). The results were statistically analyzed. A uniform coagulated layer was created on the tip portion of the K-file when the insertion depth was up to 3.0 mm. The intracanal temperature of a mandibular anterior tooth increased up to 40.8°C–45.1°C during the 1st energization. However, the root surface temperature increased by 7.1°C–7.8°C, with temperature increase per one energization being ‹0.5°C if the time interval was ›3.0 s during the subsequent energization. In the electromagnetic apical treatment system developed based on these results, the efficient generation of Joule heat and energization were possible without harmful effects on the periodontal tissue.
Keywords: Electromagnetic apical treatment; High-frequency current; Heat generation; Temperature increase; Root canal treatment
Introduction
The primary etiological cause of periapical periodontitis are preexisting substances in the root canal such as bacteria and/or their products, infected tooth or pulp debris [1-3]. Howeverachieving perfect disinfection by chemomechanical preparation is difficult owing to the root canal’s complex anatomical morphology [4,5]. Recently, a diode laser or pulsed Nd: YAG laser has been utilized [6-8]. However, such laser devices need to be improved further so that they can exhibit the crucial difficulty of directing appropriate radiation onto the curved root canal [9,10]. Although various medications show effective bactericidal actions [11,12]. There are concerns regarding the appearance of the antibiotic-resistant DNA [13,14].
To energize high-frequency current, the current density is increased at the tip and the Joule heat is produced. Substances inside the root canal or the root canal itself could be cauterized and sterilized by the Joule heat [15]. Note that the bactericidal action of the electrical energy has been investigated previously [16-19]. The bactericidal effect by electric current is known as the electricidal effect [16,17]. Whereas a similar effect along with an antimicrobial agent is defined as the “bioelectric effect [18,19]. Among the proposed theories on the bactericidal mechanism, electric current has been reported to enhance antimicrobial agents’ functions against the bacteria within the biofilm. The sole application of direct current is to exfoliate the biofilm from the surface layer [16]. However, the bactericidal effect of high-frequency current is owing to the mechanical action against the exopolysaccharide matrix. Thus, the weakening of the tissue membrane is possible by vibrating the biofilms under the current flow [17]. Assumptions such as the physical removal of biofilms by air bubbles owing to electrolysis or enhanced sensitivity owing to temperature increase within the biofilms have been previously reported [16].
In our previous study [20,21]. We applied high-frequency current against the gram-positive bacteria Streptococcus mutans, Staphylococcus intermedius, and Enterococcus faecalis and the gramnegative bacteria Fusobacterium nucleatum and Porphyromonas gingivalis; our results indicated a remarkable bactericidal effect against all types of bacteria. Moreover, the production quantity of inflammatory cytokines produced from the human monocyte cell line THP-1 cell stimulated by S. mutans was measured and found that S. mutans treated by an electric conduction controlled the production of inflammatory cytokines at the same level as S. mutans that was heattreated at 100°C for 10 min; hence, the pathogenicity of S. mutans was confirmed to be effectively inactivated. Simultaneously, the gingipain activity inhibitory action of P. gingivalis was also recognized.
Therefore, we assume that these effects are involved for effectively treating the infection source in the uninstrumented area. The traceability of electric current traveling in the intricate morphology of the root canals can be excellent; thus, its permeability in the curved or constricted root canal is expected to be high. Nevertheless, overheating owing to the high-frequency current may increase the risk of harmful effects on the periodontal tissue. Therefore, we measured the heat produced by energization around the K-file and evaluated temperature changes on the intracanal and root surfaces.
Materials and Methods
The Electromagnetic Apical Treatment (EMAT) device
The high-frequency treatment device (J. MORITA MFG. Corp., Kyoto, Japan) is monopolar and is composed of the electrosurgical main unit, active electrode, and counter electrode. The electro unit is characterized by the maximum output power of 15.5 W, the output discrete wave of 50-70 ms, and the frequency of 510 kHz.
Evaluation of coagulation by the joule heat
Energization method: As a heat-sensitive substance, according to a previously used method [22,23] an egg white was placed into a polystyrene Sample cup (5 ML, SANPLATEC Corp., Osaka, Japan) with 18.0 mm diameter and 35.0 mm depth. K-file #10–40/.02T (MANI Inc. Utsunomiya, Japan) was used as the active electrode, whereas a stainless steel wire of 1.30 mm (TOMY Inc., Tokyo, Japan) was used as the counter electrode. The current flow was energized for 1.0 s.
To investigate the influence of K-file based on its depth, K-file #10–40/.02T was inserted into the egg white every 1.0 mm from 1.0 to 16.0 mm in depth. To evaluate the Joule heat derivation aspect inside the root canal and to examine influences based on the Apical Foramen (AF) diameter, three types of Endo Training-Bloc (J 02 Taper, Dentsply MAILLFER Corp., NY, USA) with AF diameter/ taper degree of #15/.02T, #30/.02T, and #60/.02T were installed in the Sample cup 5 ML. The egg white was placed into the Sample cup 5 ML and into the root canal. K-file #10/.02T was inserted into the root canal and energized the same way as above. The current effective value at each K-file was measured using an oscilloscope (MIXED SIGNAL OSCILLOSCOPE DLM2054, Yokogawa Test & Measurement Corp., Tokyo, Japan) (Figure 1).
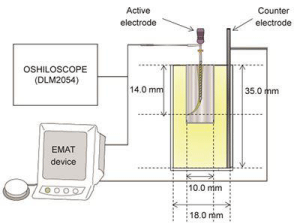
Figure 1: A schematic drawing of the experimental set up for the coagulation
by the Joule heat.
Judgment: The Joule heat generation was examined based on the coagulation of egg white on K-file. The judgment of the coagulation of egg white is defined as follows: “Code 0” indicates that the egg white did not cover K-file as observed by naked eyes, no adhesion; “Code 1” was for thin coverage; “Code 2” was for localized thick coverage; and “Code 3” was for an entirely thick coverage (Figure 2).
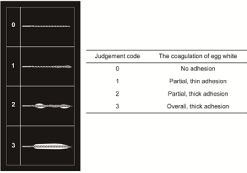
Figure 2: Judgement of the coagulation of egg white.
Influence of the energization period: K-file #10/.02T was inserted into the agar plate at a depth of about 3.0 mm and maximum output source of 15.5 W and then energized. Current flow conditions varied as follows: frequency of 510 kHz and energization periods of 0.1, 0.5, 1.0 and 1.5 s. After taking a digitized image of the surface features of the agar plate, the agar depression diameter was measured using Image J ver.1.51 (National Institutes of Health, Maryland).
Temperature of intracanal and root surfaces: The five human mandibular anterior tooth crowns were cut to obtain a 12.0-mm root length. Small holes were fabricated from the root surface to the root canal wall at 2.0, 5.0, and 8.0 mm toward the crown side from AF. Then, a thermocouple was installed and fixed using a 4META/MMATBB adhesive resin (Super Bond, Sun Medical Corp., Moriyama, Japan) within the dentin. The alginate impression material mixed with physiological saline solution was placed into the polystyrene Sample cup (5A-1, SANPLATEC Corp.); the subject tooth was implanted at a 10.0-mm depth. K-file #10/.02T was used as the active electrode and coated with parylene except for 3.0 mm at the tip portion, resulting in only the uncoated tip portion being electroconductive. After filling the root canal with a physiological saline solution, K-file was inserted 2.0 mm on the crown side from AF, followed by energizing for 1.0 s each time with energizing intervals of 1.0, 3.0, and 5.0 s. In total, we performed ten energizations. Using a data logger (midi LOGGER GL820, Graphtec Corp., Yokohama, Japan), we measured the temperatures of the intracanal and root surfaces at AF-2.0 mm, AF-5.0 mm, AF-8.0 mm. The current effective value was measured using the oscilloscope (Figure 3).
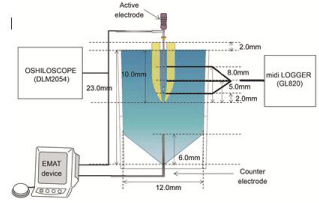
Figure 3: A schematic drawing of the experimental set up for the temperature
of intracanal and root surfaces.
Statistical analysis: Linear regression analyses were conducted to determine the correlation between the current effective value and K-file diameter, AF diameter. The agar depression diameters were compared between groups and statistically analyzed using a one-way analysis of variance with the Tukey test at a 0.05 level of significance. The statistical analysis software SPSS Statistics Base version 25.0 (SPSS Inc., Chicago, IL, USA) was used for both statistical analyses.
Results
Coagulation by the joule heat
Influence of the active electrode: All types of tip diameters exhibited “Code 3” coagulation when the insertion depth was up to 3.0 mm; however, the coagulation depth could be reduced by increasing the tip diameter (Figure 4a). The current effective value was 116–349 mA rms after inserting K-file #10/.02T into the Sample cup 5ML and was increased by increasing the insertion depth of the K-file (p ‹ 0.001). With K-file #40/.02T, the current effective value was 166–327 mA rms and showed no significant difference from that for K-file #10/.02T (Figure 4b).
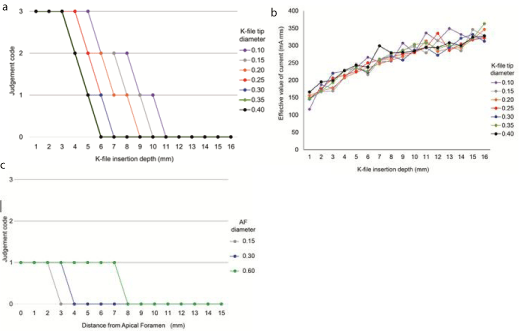
Figure 4: Factors affecting the Joule heat. (a) K-file with all types of tip diameters were with thick coverage entirely when the insertion depth was up to 3.0 mm;
(b) K-file tip diameter did not affect the current effective value; c) Coagulation was noticed at positions away from AF by increasing the scale of the AF diameter.
Influence of the AF diameter
Acoagulated layer was found at the tip portion of the K-file, and not at the apical portion with a K-file placed close to AF. By increasing the AF diameter scale, coagulation was detected at positions away from AF. In the Endo Training-Bloc, only “Code 1” was observed, and “Code 2” and “Code 3” were not detected (Figure 4c). In this experiment, the current effective value (‹10 mA rms) was measured only at the AF portion. No reading was seen when the distance from AF exceeded 1.0 mm even when using the thickest K-file (data not shown).
(a) K-file with all types of tip diameters were with thick coverage entirely when the insertion depth was up to 3.0 mm
(b) K-file tip diameter did not affect the current effective value
(c) Coagulation was noticed at positions away from AF by increasing the scale of the AF diameter
Energization period
By increasing the energization period, the agar depression diameter significantly (p ‹ 0.01) increased in a time-dependent manner up to 1.0 s (0.81 mm). However, no significant difference was observed between 1.0- and 1.5-s energizations (Figure 5a,b).
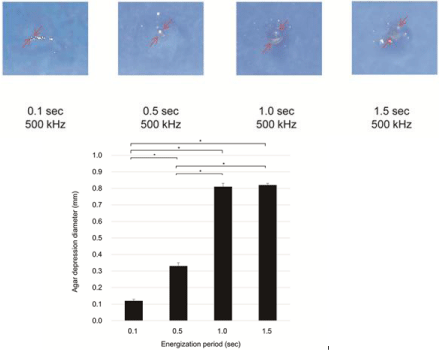
Figure 5: Agar depression by the joule heat. (a) Agar depression images; (b) Agar depression diameter.
(a)Agar depression images
(b)Agar depression diameter
Temperature of the intracanal and root surfaces
The intracanal temperature at AF-2.0 mm, AF-5.0 mm, and AF- 8.0 mm increased rapidly by 42.5, 22.3, and 0.5°C, respectively, during the first energization. However, during the subsequent energization, all temperature increases were small with the average increments being 0.7, 0.7, and 0.3°C, respectively, at each heating time (Figure 6a). The root surface temperature at AF-2.0 mm increased by 7.1– 7.8°C at the first energization; however, the temperature increase at the subsequent energization was 1.4, 0.7, and 0.5°C for flow intervals of 1.0, 3.0 and 5.0 s, respectively. Hence, only 1.0-s interval exhibited the heat reserve increase effect of ›10°C (Figure 6b).
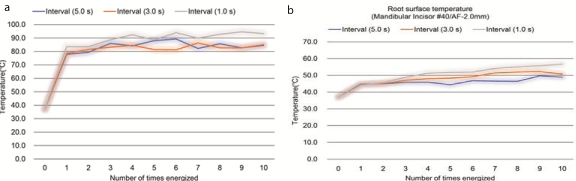
Figure 6: Temperature change. (a) The intracanal temperature increased rapidly during the first energization and was low in the subsequent energization; (b) The
root surface temperature did not exceed 45°C during the first energization and did not increase to a large extent during the subsequent energization for a time
interval of ›3.0 s.
(a) The intracanal temperature increased rapidly during the first energization and was low in the subsequent energization.
(b) The root surface temperature did not exceed 45°C during the first energization and did not increase to a large extent during the subsequent energization for a time interval of ›3.0 s.
Discussion
We demonstrated egg white coagulation at the tip portion of the active electrode, although studies [23,24] have suggested heat generation at the apical portion. The current effective value increased by increasing the K-file insertion depth (p ‹ 0.001), and no differences were observed in the K-file diameter (Figure 4b). However, we observed coagulation at K-file insertion depth of ‹3.0 mm, which became more remarkable in a smaller-diameter K-file (Figure 4a). The current effective value increased as the file insertion depth increased. However, because the coagulation of egg white decreased, the increase in the current density and contact resistance was assumed to be caused by the small contact area between the egg white and file, primarily affecting the Joule heat generation. Hence, to more effectively apply the Joule heat, the K-file diameter is should be as small as possible and the energizing zone should be within 3.0 mm; therefore, the current density can be increased.
When energizing using the Endo Training-Bloc, the heat generation feature differed from that with energizing inside a cylindershaped container, and coagulation was observed at the tip portion of the K-file only when it approached the vicinity of the AF portion. This phenomenon became more prominent when the AF diameter became smaller (Figure 4c). The Joule heat amount, according to the Joule’s first law, increases as the current increases. Moreover, according to the Ohm’s law, the current decreases as resistance increases. Additionally, the resistance is proportional to the length of the electrode and inversely proportional to the cross-sectional area. In the case of an Endo Training-Bloc, the current was energized only through AF. Therefore, smaller the diameter of AF and longer the distance up to AF, higher is the electrical resistance; consequently, no heat generation was considered when a location far from the AF was energized. Thus, to effectively generate the Joule heat, several factors, such as file diameter, insertion depth, solution amount in the root canal, and AF diameter, should be considered.
The agar depression diameter could be expanded in a timedependent manner up to 1.0 s, but was not significantly (p ‹ 0.01) different from a case of 1.5-s energization (Figure 5a,b). When heated for ›1.0 s, the electric current was not conducted and no heat was generated from the results of the evaporation of the agar. Hence, when high-frequency current is applied in a root canal, it might not be necessary to energize for ›1.0 s. In this experiment, 1.0% agar plate was employed as a sensible heat substance because the egg white adheres to the K-file.
Special caution should be taken regarding the Joule heat at the root surface when high-frequency current is clinically applied. Excess temperature increase can irreversibly damage the periodontal ligament [25,26] and unnecessary heat might scald the periodontal tissue [27,28]. Bone tissue exhibits irreversible changes on heating at 50°C for 1 min or 47°C for 5 min; thus, as bone resorption progresses, the bone is eventually replaced with adipose tissue [29]. To avoid severe bone tissue damage and decrease in regeneration function, heating condition has been determined at 44–47°C within 1 min [30]. Upper limit temperature for the periodontal tissue might be ~45°C [26]. To predict the influences on periodontal tissues, we measured intracanal and root surface temperature changes. We used the mandibular anterior tooth [31] because it has a relatively thin root dentin, which easily transfers the intracanal temperature to the root surface. When energization was intracanally conducted ten times using a K-file with the conductive area limited to the tip portion of 3.0 mm, the temperature increased only at the uncoated tip portion, e.g., to evenly cauterize a root canal wall, energization should be performed once or several times by moving the K-file rather than energizing multiple times at the same position. However, the temperature at the root surface did not exceed 45°C during the first energization, and high temperature increase was not observed during the subsequent energization for a time interval of ›3.0 s (Figure 6b). Hence, if the interval is ›3.0 s, the temperature at the root surface will not continue to overheat to ›47°C for ›1 min. The root surface temperature will possibly be lower than that in vitro because of heat diffusion by convention flow via the blood vessels within the periodontal ligament and/or heat dissipation by the heat conductivity of the periodontal ligament. The possibility of periodontal tissue damage should be considered as low; moreover, heat generation might cauterize and coagulate bacteria and infected debris because of high-frequency energization.
The following optimum conditions were identified to design and operate the EMAT system.
EMAT device
Based on egg white coagulation evaluation, K-file #10/.02T is selected as the active electrode based on the high heat generation. Considering the Joule heat generation, K-file was coated with a nonconductive material, such as parylene, except for its tip portion (3.0 mm).
EMAT procedure
After a chemomechanical root canal preparation (Figure 7a,b), a high electroconductive liquid, such as physiological saline solution or sodium hypochlorite solution, is placed into the root canal, and then energization is conducted for 1.0 s intermittently at 3.0- s intervals to prevent overheating, indicated by the root surface temperature (Figure 7c). To eliminate the scatter in the conductivity of the K-file, it should be pulled out from the root canal for each energization process, and if protein-coagulated layer adheres to the K-file, it should be cleaned using a wet gauze. In this study, the agar depression diameter was approximately 0.81 mm when the current flow was energized with 500-kHz frequency for 1.0 s into the agar plate. Hence, in cases without a periapical lesion, the point that is 1.0~2.0 mm apart from AF in the coronal direction is defined as an energization start point to avoid the direct heating of the periodontal ligament. To avoid excess heating, a 1.0-s energization is conducted 1-3 times with 1.0-mm intervals while pulling the K-file toward the crown. When a periapical lesion should directly be heat-denatured, additional energization can be performed on AF and inside the apical lesion (Figure 7d). Regarding the bactericidal effect, the Joule heat generated by high-frequency energization can denature around the K-file; thus, by applying the abovementioned EMAT system, highfrequency energization should yield the bactericidal effect in the root canal and the so-called uninstrumented area, which is considered difficult to reach during a chemomechanical root canal preparation. Moreover, this system provides a useful nonsurgical treatment method for refractory cases. Note that heat generation using the EMAT system is confirmed to cause few adverse events. Studies are required on the clinical applications and safety considerations of this system.
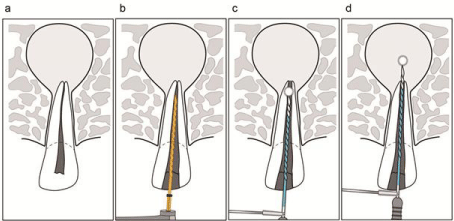
Figure 7: The electromagnetic apical treatment procedure.
(a) Apical periodontitis with periapical lesion (b) Chemomechanical root canal preparation (c) 1.0-s energization in root canal 1–3 times with 1.0-mm intervals (d)
Additional energization on AF and inside the apical lesion when necessary.
In conclusion, the EMAT system can be used to generate Joule heat using K-file coated, except for its tip portion. The energization method with ›3.0-s interval prevented temperature increase up to the heat resistance limit of the periodontal tissue.
(a) Apical periodontitis with periapical lesion
(b) Chemomechanical root canal preparation
(c) 1.0-s energization in root canal 1-3 times with 1.0-mm intervals
(d) Additional energization on AF and inside the apical lesion when necessary
Acknowledgement
The author would like to express sincere appreciation to Yoshiki Oshida, Professor Emeritus, Indiana University School of Dentistry for helping us with the article’s structure and Kazunari Matoba at J. Morita MFG. Co. for providing the high-frequency device. The authors deny any conflicts of interest related to this study.
Compliance with Ethical Standards
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (The Hokkaido University Faculty of Dental Medicine Clinical and Epidemiological Research Ethics Committee, Approval No. 2018-7) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats Oral Surg Oral Med Oral Pathol. 1965; 20: 340-49.
- Fabricius L, Dahlén G, Ohman AE, Möller AJ. Predominant indigenous oral bacteria isolated from infected root canal after varied times of closure Eur J Oral Sci. 1982; 90: 134-44.
- Nair PNR. Light and electron microscopic studies of root canal flora and periapical lesions J Endod. 1987; 13: 29-39.
- Vertucci FJ. Root canal morphology and its relationship to endodontic procedures Endod Topics. 2005; 10: 3-29.
- Ahuja P, Nandini S, Ballal S and Velmurugan N. Effectiveness of four different final irrigation activation techniques on smear layer removal in curved root canals: a scanning electron microscopy study J Dent. 2014; 11: 1-9.
- Cheng X, Chen B, Qiu J, He W, Lv H, Qu T, et al. Bactericidal effect of Er: YAG laser combined with sodium hypochlorite irrigation against Enterococcus faecalis deep inside dentinal tubules in experimentally infected root canals J Med Microbiol. 2016; 65: 176-87.
- Wang QQ, Zhang CF, Yin XZ. Yin. Evaluation of the bactericidal effect of Er,Cr: YSGG, and Nd: YAG lasers in experimentally infected root canals J Endod. 2007; 33: 830-32.
- Arnabat J, Escribano C, Fenosa A, Vinuesa T, Gay-Escoda C, Berini L, et al. Bactericidal activity of erbium, chromium: yttrium-scandium-gallium-garnet laser in root canals Lasers Med Sci. 2010; 25: 805-810.
- Kimura Y, Wilder-Smith P, Matsumoto K. Lasers in endodontics: a review Int Endod J. 2000; 33: 173-85.
- Hülsmann M, Peters OA, Dummer PMH. Mechanical preparation of root canals: shaping goals, techniques and means Endod Topics. 2005; 10: 30- 76.
- Alam T, Nakazawa F, Nakajo K, Uematsu H, Hoshino E. Susceptibility of Enterococcus faecalis to a combination of antibacterial drugs (3Mix) in vitro J Oral Biosci. 2005; 47: 315-20.
- Nakornchai S, Banditsing P, Visetratana N. Clinical evaluation of 3Mix and Vitapex as treatment options for pulpally involved primary molars. Int J Paediatr Dent. 2010; 20: 214-21.
- Jungermann G B, Burns K, Nandakumar R, Tolba M, Venezia R A, Fouad A F. Antibiotic resistance in primary and persistent endodontic infections J Endod. 2011; 37: 1337-44.
- Rôças IN, Siqueira JF Jr. Detection of antibiotic resistance genes in samples from acute and chronic endodontic infections and after treatment Arch Oral Biol. 2013; 58:1123-28.
- W. Klein. Einige Mitteilungen uber die Anwendung des Diathermiestromes in der Zahnheilkunde Z F Stomat. 1929; 27: 443.
- Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms. A systematic review Int J Artif Organs. 2008; 31: 786-95.
- R Caubet, F Pedarros-Caubet, M Chu, E Freye, M de Belém Rodrigues, JM Moreau, et al. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms Antimicrob Agents Chemother. 2004; 48: 4662- 4664.
- Rediske AM, Roeder BL, Brown MK, Nelson JL, Robison RL, Draper DO, et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model Antimicrob Agents Chemother. 1999; 43: 1211-1214.
- Peterson RV, Pitt WG. The effect of frequency and power density on the ultrasonically enhanced killing of biofilm-sequestered Escherichia coli Colloids Surf B Biointerfaces. 2000; 17: 219-27.
- T. Tominaga. Application of electro-magnetic wave to endodontic treatment– EMAT (Electro-Magnetic Apical Treatment) Shikoku Dent Res. 2011; 24: 1-31.
- Tominaga T, Kitaike K, Tada E, Takahira K, Bandoh N, S. Hirao , et al. Application of electromagnetic stimulation to apical periodontitis J Jap Endod Ass. 2017; 38: 36-47.
- Tomura J. Experimental study on the pulp and root canal treatment by means of high frequency current J Stomatol Soc Jpn. 1956; 23: 44-64.
- Tomura J, Osada T. Experimental study on the pulp treatment by means of high frequency current (animal experiment) J Stomatol Soc Jpn. 1957; 24: 24-39.
- Flohr E, Flohr W. Die Anwendung der Diathermie in der Zahnheilkunde: ein Leitfaden für die Praxis Berlin: Verlag von Hermann Meusser. 1930.
- Gottlieb BU, Orban B. Veranderungen im periodontium nach chirurgischer diathermie Z F Stomat. 1930; 28: 1208.
- Levy M. Kombinierte anwendung physic kalischer behandlungsmethonden bei pulpa und wurzelerkrankunge. Zarzt Rdsch. 1930; 39: 884.
- Lundskog J. Heat and bone tissue Scand J Plast Reconstr Surg. 1972; 9: 1-80.
- Eriksson AR, Albrektsson T, Grane B, McQueen D. Thermal injury to bone: a vital-microscopic description of heat effects Int J Oral Surg. 1982; 11: 115- 121.
- Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital microscopic study in the rabbit J Prosthet Dent. 1983; 50: 101-107.
- Eriksson AR, Albrektsson T. The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber J Oral Maxillofac Surg. 1984; 42: 705-711.
- Vijayalakshmi BH, Girija SS, Padmaja M. An ex-vivo evaluation of thermal changes in periodontal ligament during the use of thermoplasticised gutta percha obturating techniques Int J Recent Sci Res. 2015; 6: 4056.