
Research Article
J Dent & Oral Disord. 2021; 7(1): 1156.
Metaplastic Changes in Odontogenic Cysts of the Jawbone: Their Significance and Relation to MUC Family Expression
Harada H1,2*, Iizuka N1, Nakatsuka SI3, Shima M4, Date E1, Honma K5 and Kurose A2
1Department of Diagnostic Pathology, Kishiwada City Hospital, Japan
2Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, Japan
3Department of Pathology, Sakai City Medical Center, Japan
4Department of Oral Surgery, Kishiwada City Hospital, Japan
5Department of Diagnostic Pathology and Cytology, Osaka International Cancer Institute Hospital, Japan
*Corresponding author: Hiroshi Harada, Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, 5 Zaifu, Hirosaki 036-8562, Japan
Received: February 22, 2021; Accepted: March 17, 2021; Published: March 24, 2021
Abstract
Odontogenic cysts are typical diseases that account for the majority of lesions that occur in the jawbone, among which radicular and dentigerous cysts are the most common. In some cases, metaplastic changes in these cysts result in the development of goblet-like mucous cells admixed with ciliated columnar cells. Odontogenic cysts are of little importance to emergent risk in normal conditions, but such histological alterations could be drastic and even confusing. We examined the expression of MUC family in these cystic lesions and investigated their relationship with histomorphological features. 6 cases of radicular and dentigerous cysts with obvious metaplastic changes were studied. Of these, 4 were male and 2 were female. One case occurred in the maxilla and 5 in the mandible. Immunohistochemically, almost the entire epithelium was positive for MUC1 and MUC4 in all cases, and the decapitation-like protrusions on the surface layer showed stronger expression. MUC5AC was selectively expressed in mucous cells, while MUC2 and MUC6 were negative. The lining epithelium with metaplastic changes closely resembled the bronchial epithelium, and MUC expression indicates a potential role in these morphological changes.
Keywords: Radicular cyst; Dentigerous cyst; Mucous cell; Ciliated cell; Metaplasia; MUC
Introduction
Odontogenic cysts are typical diseases that account for the majority of lesions that occur in the jawbone, among which radicular and dentigerous cysts are the most common. As a result of metaplastic changes, both types occasionally display goblet-like mucous cells mixed with ciliated columnar cells. In such cases, the histopathological diagnosis may be complicated by this unusual morphology.
MUC is a general term for the core mucus proteins, and many types have been identified. Several family members are currently used as prognostic factors in various cancer types, but to our knowledge, their expression in odontogenic cysts has not been described except our reports [1,2].
In this study, we examined the expression patterns of five MUC family members in these cystic lesions and investigated their association with histomorphological features of odontogenic cysts.
Patients and Methods
Six cases of radicular and dentigerous cysts with obvious metaplastic changes in four men and two women were extracted from the diagnostic registration files at Kishiwada City Hospital from 2014 to 2018. One case occurred in the maxilla and five occurred in the mandible. The patient age at the time of diagnosis was 36 to 71 years (average, 44 years). Both radicular cysts had developed in relation to the first molars, whereas all four dentigerous cysts were lesions caused by the third molars.
Formalin-fixed paraffin-embedded specimens were used for light-microscopic observation by conventional methods. Samples were fixed in 10% formalin, embedded in paraffin, cut into 4μm-thick serial sections, and used for hematoxylin and eosin stain, and also histochemical stains, such as Periodic Acid–Schiff (PAS) and alcian blue stains on demand.
Immunohistochemical analyses were performed for MUC1, MUC2, MUC4, MUC5AC and MUC6 with similar serial sections. The procedure was performed using the Roche BenchMark ULTRA IHC/ ISH Staining Module (Ventana Medical Systems, USA) according to the manufacturer’s instructions. Data of primary antibodies used for immunohistochemistry was as follows: MUC1 (clone H23, prediluted, Ventana Medical System, USA), MUC2 (clone MRQ-18, prediluted, Sigma-Aldrich Co, USA), MUC4 (clone EPR9308, 1:50 dilution, Abcam, USA), MUC5AC (clone MRQ-19, prediluted, Sigma-Aldrich Co, USA) and MUC6 (clone MRQ-20, prediluted, Sigma-Aldrich Co, USA).
Two specimens of normal oral mucosa excised during the treatment of other diseases were used as control specimens in addition to three radicular and dentigerous cysts without obvious metaplastic changes.
Results
Light-microscopic observation
Histologically, all lesions preserved the basic structure of the cyst and consisted of a cystic wall of fibrous connective tissue and a non-keratinizing stratified squamous epithelium covering the luminal surface (Figure 1a). Inflammatory infiltration in the wall was relatively minor, not only in dentigerous cysts, which are not caused by inflammation, but also in radicular cysts.
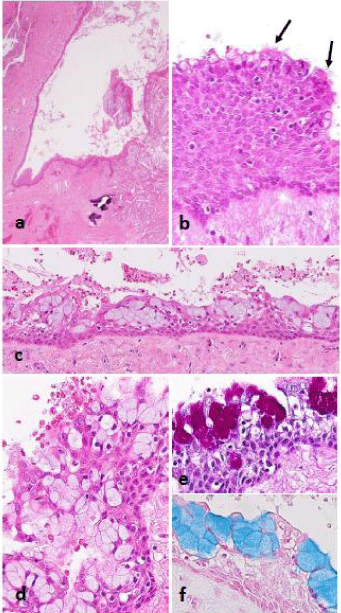
Figure 1: Histopathological findings. a,b: Normal section of a dentigerous
cyst lined by squamous cells with obvious intercellular bridges. Sporadic
mucous cells are seen in superficial layer as well as a few ciliated cells
(arrows). c,d: Numerous mucous cells are evident in the lining epithelium
in other areas; e,f: In PAS and alcian blue stains, the positive reactions
resemble mucoepidermoid carcinoma.
Goblet-like mucous cells were present in varying proportions within the lining epithelium (Figure 1b-1d), and protrusions into the lumen reminiscent of decapitation were also observed in areas where no definite mucous cells had been produced (Figure 2a and 2c); both of which were positive for PAS and alcian blue stains (Figure 1e,1f and 2b,2d). The number of mucous cells in the lining epithelium was notably higher than that of the ciliated epithelium, but a certain number of ciliated epithelium were evident somewhere in most cases (Figure 1b and 2c).
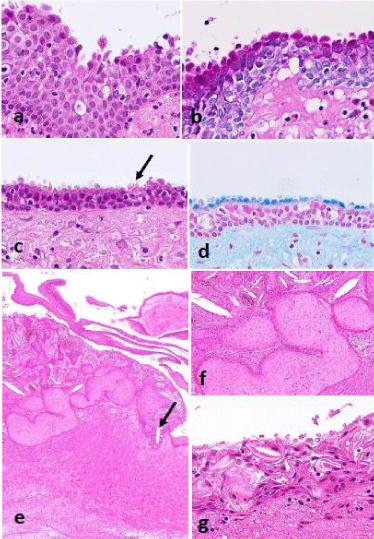
Figure 2: a-d: A case of radicular cyst associated decapitation-like
appearance; c: Arrow designating superficial cilia; b,d: Decapitation-like
protrusions showing positive reaction for PAS and alcian blue stains; e,f:
Another case of dentigerous cyst associated with nesting proliferation of nonkeratinizing
squamous cells equivalent to squamous odontogenic tumor-like
proliferation. Arrow designating a focus including mucous cells; g: Same case
including hyaline bodies in the lining epithelium.
In a case of dentigerous cyst, there were found nests composed of bland non-keratinizing squamous cells within the thickened cystic wall beneath or contacting the lining epithelium, which was interpreted as a squamous odontogenic tumor-like proliferation (Figure 2e and 2f).
The lining epithelium occasionally involved hyaline bodies, which are particular to odontogenic cysts (Figure 2g).
Immunohistochemistry
Almost the entire epithelium was positive for MUC1 and MUC4 in all cases, and the decapitation-like protrusions on the surface layer showed stronger expression in areas where no mucous cells had been produced (Figure 3a-3e). MUC5AC was selectively expressed in mucous cells and exhibited positive expression in the cytoplasm (Figure 3a-3d), but there was almost no expression in other cellular regions. MUC2 and MUC6 were negative in all areas. Similarly, the mucosal epithelium was variably positive for MUC1 and MUC4 in the two oral mucosa controls, and MUC5AC lacked any obvious expression. MUC2 and MUC6 were also negative. Similar results were obtained in radicular and dentigerous cysts without obvious metaplastic changes.
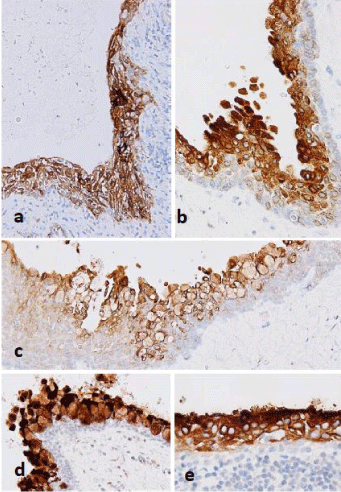
Figure 3: Immunohistochemistry. The other case of radicular cyst. a-c:
MUC1 showing a wide range of expression including decapitation-like
protrusions and mucous cells; d,e: Similar expression of MUC4 is found in
other dentigerous cysts.
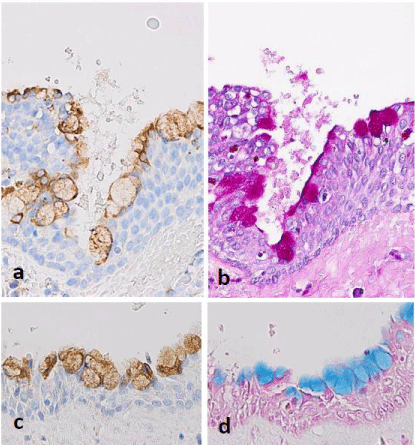
Figure 4: Immunohistochemistry. Another case of dentigerous cyst. a,c:
MUC5AC selectively expresses in mucous cells; b,d: Corresponding positive
reactions in PAS and alcian blue stains.
Discussion
Metaplastic changes and differential diagnoses
Metaplastic changes producing mucous cells admixed with ciliated epithelium may appear in odontogenic cysts to varying degrees, and this must be fully recognized for accurate diagnosis. Similarly, ameloblastoma, one of representative odontogenic lesions, are extremely rarely associated with mucous cells [3,4]. In such cystic lesions, the differential diagnosis mainly includes glandular odontogenic cyst and central mucoepidermoid carcinoma of the jawbone, both of which differ from radicular and dentigerous cysts in their clinical behavior. Notably, mucoepidermoid carcinoma is generally a low-grade but definite carcinoma, and special care must be taken in its differentiation.
In glandular odontogenic cysts, the lining epithelium is composed of columnar cells, and microcysts, which are luminal structures usually composed of columnar cells but not mucous cells, are conspicuous within the thickened lining epithelium. Such findings were absent in the present series at all. According to the current 4th edition of World Health Organization (WHO) classification, it is extremely rare for glandular odontogenic cysts to take a dentigerous image, and the adjacent roots may be accompanied by exclusion or absorption. Glandular odontogenic cysts differ from dentigerous or radicular cyst in these features.
Central mucoepidermoid carcinoma is a unique salivary glandtype tumor that occurs in the jawbone. But intercellular bridges characteristic of squamous cells were evident throughout the samples in the present series, and few components corresponded to intermediate cells. In such cases, it is inappropriate to interpret the lesion as a mucoepidermoid carcinoma simply because it contains both squamous and mucous cells [1,2].
MUC family expression
In the present series, almost the entire epithelium was immunohistochemically positive for MUC1 and MUC4, with MUC5AC selectively expressed in mucous cells, while MUC2 and MUC6 were negative. We observed similar cases in the consultation case series of the primary author (HH), and obtained comparable results to five cases of radicular or dentigerous cyst, including two previously reported [1,2]. To our knowledge, no analysis of MUC family expression in odontogenic cysts been described in the literature except ours.
Regarding non-odontogenic cysts, Menditti et al. reported that goblet cells involved in a nasolabial cyst were positive for MUC2 and MUC5AC, similar to the human lacrimal organs, and speculated that it was a developmental non-odontogenic cyst of the soft tissue originating from the lower portion of the naso-lacrimal duct [5]. However, they did not consider the ciliated epithelium involvement in the lining epithelium.
Conversely, López-Ferrer et al. reported that MUC1 and MUC4 are consistently expressed in the normal bronchial mucosa and MUC2 and MUC5AC are selectively expressed in mucous cells [6]. Considering that the rate of positive MUC2 expression is rather low, that MUC1, MUC4, and MUC5AC were positive in all cases, and that in most cases there is slight ciliated epithelium intervention, these metaplastic changes are consistent in reflecting the characteristics of the bronchial epithelium. Regarding the pattern of expression, the membrane-bound type mucin is predominant over the secretory type mucin, as would be predicted because the former is confined to the mucosal surface and plays a role in protecting the mucosa. MUC5AC, which is not expressed in normal mucosa, is a secretory type mucin and may assist the function of the ciliated epithelium.
Embryological aspect and relation to the respiratory tract epithelium
Furthermore, the odontogenic epithelium is embryologically derived from the mucosal epithelium of the oral cavity, the latter of which is not dissimilar to the respiratory epithelium in its developmental background [7]. Thus, odontogenic cysts and the respiratory epithelium are not completely unrelated, and such an unusual morphology can be interpreted as metaplastic changes that mimic the differentiation of neighboring organs [1]. In fact, sinonasal ameloblastomas have been reported [8], and the authors experienced a similar case [7]. In both instances, it was understood that the tumors occurred from the sinonasal mucosa with squamous metaplasia, and it was suggested that even metaplastic squamous cells of the sinonasal tract could produce an odontogenic lesion. At the same time, odontogenic lesions such as radicular and dentigerous cysts in the present series followed the reverse route to promote metaplastic changes that mimic the characteristics of the respiratory system.
MUC family expression has also been examined in mucoepidermoid carcinoma of the salivary glands [9,10]. Although consistent expression of MUC1 and MUC4 was observed, MUC5AC and MUC6 exhibited large variability and thus were unsuitable for the differentiation of cystic lesions, as in the present series. In addition, the relationship between their expression pattern and prognosis is emphasized, rather than the nature of mucus itself, akin to cancers of the digestive tract [11,12] or other cancer types [13]. However, these cysts are benign lesions and not tumors, and hence there is no significance in this respect.
In the current study, inflammatory infiltration in the cystic wall was relatively minor in most cases, even in radicular cysts, and thus it was likely accounted for the lining epithelium escaping exfoliation, with many opportunities to cause metaplastic changes as well as to produce many hyaline bodies. The mechanism by which the metaplasia of mucous cells and ciliated epithelium occurs in odontogenic cysts is unclear, but long-term continuous contact with certain content fluids confined to the occluded space could stimulate the lining epithelium, triggering these metaplastic changes.
Odontogenic epithelium is derived from the mucosal epithelium of the oral cavity, the development of which is not dissimilar to the bronchial epithelium. It is assumed that the inner lining epithelium is an “imitation mucosa,” and thus the lining epithelium may have an enhanced defense function by promoting metaplasia, actually regarded as primitive “throwback,” to protect itself from irritation by the contents. As a result, metaplasia can also be regarded as a reasonable response to inflammatory damage to benefit the host, providing it can avoid rupture of the cystic structure.
Radicular and dentigerous cysts are common diseases of the jawbone and are not considered as emergencies under normal conditions, but such histological alterations could be drastic and even confusing [1,2]. We believe further study and exact recognition of such unique cases are of importance.
Squamous odontogenic tumor-like proliferation closely resembles squamous odontogenic tumor and rarely occurs within the cystic wall of various odontogenic cysts [14]. As being a non-neoplastic process, it is regarded to have little importance in the respect of clinical behavior. Both radicular and dentigerous cysts are involved, and the former more frequently [15].
Conclusion
We investigated the morphological changes in odontogenic cysts of the jawbone accompanied by metaplastic changes including mucous cells and ciliated epithelium, and revealed that MUC family proteins exhibited uniform expression patterns in all examined cases. But, they have no significance as a prognostic factor because cysts are benign lesions, and they cannot be used for the differentiation from salivary mucoepidermoid carcinomas, which have a large variation in MUC expression.
However, lining epithelium with metaplastic changes has been shown to closely resemble the bronchial epithelium, and it is a quite interesting finding when considering the developmental background related to neighboring organs and on basis of morphology in conjunction with MUC family expression.
Ethical Approval
Obtained with Kishiwada City Hospital Approval #33.
Note: In addition to being a research fellow at Hirosaki University Graduate School of Medicine, the primary author (HH) is also a visiting staff pathologist in Kishiwada City Hospital.
References
- Harada H, Kihara T, Abe H, Kawahara A, Akiba, Kurose A. Dentigerous cyst exhibiting prominent mucous cell metaplasia: report of a unique mandibular case mimicking mucoepidermoid carcinoma. Med Mol Morphol. 2021.
- Harada H, Marutsuka K, Abe H, Kawahara A, Akiba J, Kurose A. Mucous and ciliated cells in oral lesions: potential pitfall of additional two cases. Clin Onco. 2021; 4: 1-5.
- Wilson D, Walker M, Aurora N, Moore S. Ameloblastoma with mucous cell differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91: 576-578.
- Punnya AV, Rekha K. “Ameloblastoma with mucous cells”: review of literature and presentation of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106: e20-26.
- Menditti D, Laino L, DI Domenico M, Troiano G, Guglielmotti M, Sava S, et al. Cysts and pseudocysts of the oral cavity: Revision of the literature and a new proposed classification. In vivo. 2018; 32: 999-1007.
- López-Ferrer A, Curull V, Barranco C, Garrido M, Lloreta J, Real FX, et al. Mucins as differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. Am J Respir Cell Mol Biol. 2001; 24: 22-29.
- Harada H, Kimura S, Kimura Y, Higaki K, Kurose A. Sinonasal ameloblastoma: A case report focusing on histogenesis and related morphological characteristics. Oral and Maxillofacial Surgery Cases. 2020; 6: 100201.
- Schafer DR, Thompson LD, Smith BC, Wenig BM. Primary ameloblastoma of the sinonasal tract: a clinicopathologic study of 24 cases. Cancer. 1998; 82: 667-674.
- Alos L, Lujan B, Castillo M, Nadal A, Carreras M, Caballero M, et al. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005; 29: 806-813.
- Handra-Luca A, Lamas G, Bertrand JC, Fouret P. MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am J Surg Pathol. 2005; 29: 881-889.
- Senapati S, Sharma P, Bafna S, Roy HK, Batra SK. The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol Histopathol. 2008; 23: 1541-1552.
- Shibahara H, Higashi M, Koriyama C, Yokoyama S, Kitazono I, Kurumiya Y, et al. Pathobiological Implications Of Mucin (MUC) expression in the outcome of small bowel cancer. PLoS One. 2014; 9: e86111.
- Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004; 122: 61-69.
- Wright JM. Squamous odontogenic tumorlike proliferations in odontogenic cysts. Oral Surg Oral Med Oral Pathol. 1979; 47: 354-358.
- Parmar RM, Brannon RB, Fowler CB. Squamous odontogenic tumor-like proliferations in radicular cysts: a clinicopathologic study of forty-two cases. J Endod. 2011; 37: 623-626.