
Research Article
J Dent & Oral Disord. 2021; 7(2): 1159.
Are Stem Cells Useful in the Regeneration and Repair of Cartilage Defects in the TMJ Condyle? An In Vivo Study
Guastaldi FPS1*, Hakim MA2#, Liapaki A1, Lowe B1, Faquin WC3, Thamm JR1 and McCain JP1
1Department of Oral and Maxillofacial Surgery, Massachusetts General Hospital, Harvard School of Dental Medicine, Boston, MA, USA
2Department of Oral and Maxillofacial Surgery, University of Michigan, Ann Arbor, MI, USA
3Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
#Contributed Equally
*Corresponding author: Fernando Pozzi Semeghini Guastaldi, Skeletal Biology Research Center, Department of Oral and Maxillofacial Surgery, Massachusetts General Hospital, Harvard School of Dental Medicine, 50 Blossom St, Thier Research Building, 513A, Boston, MA 02114, USA
Received: March 30, 2021; Accepted: April 14, 2021; Published: April 21, 2021
Abstract
Temporomandibular Joint (TMJ) disorders affect up to 10-40% of the population and if left untreated, may eventually lead to Osteoarthritis (OA) of the TMJ. In vivo TMJ repair and regeneration has received significant attention and represents a promising approach for the treatment of degenerative TMJ disorders. The aim of this study was to present a pre-clinical mouse model of TMJ articular cartilage defect and evaluate the utility of a potential tissue engineered TMJ therapy utilizing Mesenchymal Stem Cells (MSCs) derived from mice condyle, hydrogel, and biosilica. C57BL/6 mice (n=30) were equally divided into the following groups: sham group (S-group); control group, condylar cartilage defect only (CD-group); experimental group, condylar cartilage defect + direct administration of MSCs+hydrogel+biosilica (H-group). Mice were euthanized at 4 (n=15) and 8 (n=15) weeks and TMJ joint specimens were harvested for analysis. H&E and Safranin O stained sections showed intact articular surfaces on the condyle and glenoid fossa at both time points, maturation and distribution of chondrocytes along the condyle for the H-group compared to the CD-group. Data from this preliminary study shows that MSCs+hydrogel+biosilica may represent an experimental therapeutic compound for TMJ condylar cartilage regeneration.
Keywords: Temporomandibular joint; Tissue engineering; Hydrogel; Biosilica; Stem cells
Introduction
The Temporomandibular Joint (TMJ) plays a pivotal role in the movement coordination of the jaw during daily basic functions (i.e. speech, swallowing, eating). TMJ is a bilateral synovial joint composed of muscles, ligaments, the fibrocartilaginous articular surfaces of the mandibular condyle and glenoid fossa as well as the cartilaginous articular disc [1]. It is estimated that Temporomandibular Joint Disorders (TMDs) affect up to 10-40 % of the population, mainly young adults under 45 years of age with a sex ratio of 4:1 (women:men) or more among clinical cases of TMD pain [2], and among them 10% suffer from Osteoarthritis (OA) in the TMJ [3].
Left untreated, TMDs may eventually lead to OA of the TMJ [3]. TMJOA is typically a slowly progressive, inflammatory disease resulting in the degeneration of articulating tissues of both cartilage and subchondral bone [4] and presents either as asymptomatic1 or characterized by pain, limited function, and crepitus or clicking sounds [1,4,5].
Within the normal and healthy joint, the articular cartilage is indispensable for the smooth movements of the mandible coupled with the temporal bone bilaterally, guaranteeing a painless motion [3,6]. Clinical studies have reported that injury to the disc might be the most prevalent causative factor of TMJOA [7].
Several studies had effectively produced a degeneration resembling the OA by creating a defect on either the articular disc or the articular surface of the condyle. A partial discectomy on a mouse model and a disc perforation on rabbits, resulted in articular cartilage degeneration in both cases and early-onset of OA [7,8]. The latter model demonstrated also a heterotopic ossification in some animals with injured disc after 2-3 months [8]. Partially and totally removal of the condylar fibrocartilage in mice have been shown in recent publications to induce ectopic bone tissue and osteophyte formation [9], hyperplasia of the affected condyle along with ectopic bones and cartilage in the periarticular region leading to the development of Traumatic TMJ Ankylosis (TTMJA) [10,11].
In our study, we present a pre-clinical mouse model of TMJ articular cartilage defect and investigate a possible tissue engineered therapy for fibrocartilage regeneration. To this end, the authors test the efficacy of a hydrogel-biosilica compound, seeded with Mesenchymal Stem Cells (MSCs) derived from the mice condyle, which was applied on the site of the defect immediately after the injury. The authors hypothesize that the defects treated with the compound will improve the regeneration of the defects compared to the untreated group.
Materials and Methods
Animals
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the Massachusetts General Hospital (Protocol # 2017N000086). Thirty-six C57BL/6 mice (n=36), females, 8-10 weeks old were obtained from Charles River Laboratories (Wilmington, MA). Mice were maintained in a virus and parasitefree barrier facility and exposed to a 12-hour light/dark cycle.
Seeding of biosilica incorporating hydrogel
TMJ condyles of two mice (mice carcasses) were explanted in a sterile manner (n=4). Mesenchymal Stem Cells (MSCs) were harvested, isolated and expanded for 2 weeks to reach approximately 90% confluency. Cells were harvested at a cell viability rate over 90%. Then they were passaged, and we used the second passage of cells for the in vivo study. These steps followed standard MSCs culture method and kept under standard culture conditions as previously described [12]. Thamm et al. (2021; unpublished data) [12] proved that these cells were MSCs using flow cytometry (FACS) analysis.
The gelatine/biosilica-based hydrogel was prepared as follows: 1g of type A gelatin (Sigma-Aldrich, St. Louis, MO), sterilized under UV light for 15 minutes, was mixed with 10mL of sterile PBS in a glass beaker, previously cleaned with distilled water and exposed to UV light. After stirring for 1 hour at 40°C on a hot plate, 0.07g of functionalized biosilica nanoparticles [13,14] were added. The hydrogel containing biosilica nanoparticles was kept stirring for another 1 hour. After receiving a homogenous fluid mixture, the hydrogel was transferred into a sterile tube and placed into a water bath to adjust hydrogel temperature to 37°C. MSCs were added at a ratio of 5x106 cells/mL to hydrogel by gently pipetting up and down to obtain a homogenous cell seeded viscous hydrogel. Finally, 10% w/v hydrogel containing 1x106 cells/mL was aspirated in a sterile syringe and placed in the incubator at 37°C prior application.
Cell viability assay and DAPI (4',6-Diamidin-2-phenylindol) staining
To show cell viability after preparing the compound (hydrogel+biosilica+MSCs) and before delivery at the condylar cartilage defects in mice the following analysis were performed.
Live/dead staining kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer’s instructions. Briefly, after thawing, 1mL of Live Green (Comp. A, 1μM) was added to 1μL of Dead Red (Comp. B) and mixed with Dulbecco’s Modified Eagle Medium (DMEM), to achieve a total of 2mL working solution. Gel droplets were placed into a 6-well-plate, cut into half and were subsequently covered with working for 15 minutes at room temperature in dark. Cells were imaged under fluorescence microscope. After observing cell viability, the same hydrogel specimens were fixed in 10% neutralbuffered formalin for 1 hour, followed by washing and subsequently applying DAPI working solution. Samples were left incubating in dark for 7 minutes at room temperature. DAPI staining was finally evaluated under fluorescence microscope.
Surgical procedure
The anatomy, the surgical technique and the perioperative care of all mice followed a detailed description described by Hakim et al. [15].
Briefly, mice were anesthetized with inhalant isoflurane (Isoflurane USP, Patterson Veterinary, Greeley, CO, USA) and remained under anesthesia during the procedure. The hair of the mouse was removed under sedation and the surgical area was disinfected with Betadine solution (Purdue Pharma, L.P, Stamford, CT, USA). Mice were divided into the following groups: (I) sham group (n=10), the TMJ was opened and closed without creating any defect (S-group); (II) control group (n=10), the TMJ was opened and a linear condylar cartilage defect was created with a no 12 surgical scalpel (CD-group); (III) experimental group (n=10), created defect on condylar cartilage, similar to the previously described manner and hydrogel+biosilica+MSCs (20μl) was directly administered (H-group). After creating the defect in the mandible condyle, the hydrogel was administered over the defect and the TMJ capsule was immediately sutured to keep the hydrogel in place. The joints were not immobilized. Mice were provided soft diet during the first 72 hours post-operatively to minimize any load on their jaws. The mice were checked daily for the first 4 days post-surgery and then every 3 days to assess their health progress and wound healing. All mice of each group were euthanized at either 4 (n=15) or 8 weeks (n=15) after surgery (Figure 1).
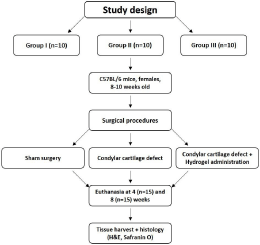
Figure 1: Flowchart depicting the workflow of the study.
Histological processing
Four and eight weeks after surgery, mice were euthanized by an anesthetic overdose of carbon dioxide (CO2) and the TMJ specimens were harvested and placed in 10% neutral buffered formalin (EMD Millipore, Burlington, MA). The samples were then decalcified in CALEX decalcifying solution containing HCL (Fisher Scientific, Pittsburgh, PA) for 2 weeks, embedded in paraffin, and cut in the sagittal plane at 5μm thickness. Sections were stained with Hematoxylin and Eosin (H&E) and Safranin O/fast green for further analysis.
Results
MSCs culturing and hydrogel preparation
Cultured cells showed the typical spindle-shape of MSCs and grew to 60 - 80% confluency within 14 days, forming viable colonies. Cells of first Passage (P1) were successfully harvested and assessed for viability rate over 95%, using an automatic cell counting machine (Bio-Rad, Hercules, CA).
Hydrogel was obtained at a cell density of 1x106 cells/mL, live/ dead and DAPI staining showed viable cells and homogenous cell distribution, incorporated within the hydrogel prior to in vivo implantation (Figure 2).
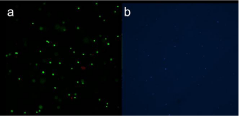
Figure 2: a) Live/dead and b) DAPI staining showed viable cells and
homogenous cell distribution of the hydrogel prior to in vivo implantation.
Surgical outcomes
Thirty-six mice underwent surgery and 6 were euthanized during their postoperative recovery, leaving 30 mice for histological evaluation (10 sham, 10 defect/control and 10 defects+hydrogel+biosilica+MSCs/experimental). Two (2) mice from the CD-group (condylar cartilage defect only) were euthanized (both due to wound dehiscence). Four (4) mice from the H-group (condylar cartilage defect+direct administration of MSCs+hydrogel+biosilica) were euthanized (2 due to keratitis, 1 was found dead and 1 due to wound dehiscence). No mice were euthanized from the S-group (Sham group). The most common intraoperative complication was bleeding. With avoidance of the posterior sigmoid notch and the facial vein, bleeding was minimal and was controlled with pressure using cotton tip applicators.
Postoperatively, there were two common complications that lead to euthanizing the mice: 1) wound dehiscence and 2) eye ulceration leading to euthanasia. Wound dehiscence was minimized by careful closure technique and adequate post-operative pain control to reduce the risk of mice scratching the wounds. Eye ulceration risk was minimized by repeatedly applying lubricant to the ipsilateral eye and protecting the facial nerve during surgery to allow for eye blinking/ protective reflect, and by applying artificial tears postoperatively. More extensively described by Hakim et al. (2020) [19].
Histological analysis
All samples were histologically evaluated by an experienced head and neck pathologist. Due to difficulty sectioning and obtaining slides, the most representative specimens from each group were selected and, therefore, included in the histologic interpretation. Two sham slides, at 4 and 8 weeks, showed an intact condylar outline with haphazard distribution of predominantly mature chondrocytes (Figure 3a and 3c). Two CD slides, one at 4 weeks (Figure 4a) and one at 8 weeks (Figure 4c), showed increased numbers of immature chondrocytes in an irregular distribution near the condylar surface. Safranin O stains highlighted the increased numbers of chondrocytes in the 4-week CD sections (Figure 4b). In addition, degenerative changes including the condyle surface and the glenoid fossa were present and seen especially in the 8-week section (Figure 4d). The H slides, showed intact articular surfaces on the condyle and glenoid fossa at both 4 and 8 weeks (Figure 5a and 5c), and there was maturation and an even distribution of chondrocytes along the condyle contrasting with the pattern seen in the sham group (Figure 5b and 5d).
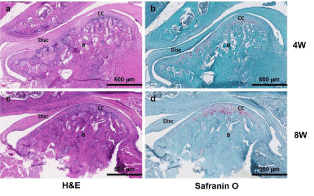
Figure 3: Histological analysis. H&E and Safranin O staining of the sham
group (S-group) at 4- and 8-week time points. B: Bone; CC: Condylar
Cartilage. Scale bar: 500μm.
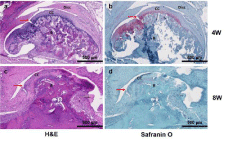
Figure 4: Histological analysis. H&E and Safranin O staining of the control
group (CD-group) at 4- and 8-week time points. B: Bone; CC: Condylar
Cartilage; Red arrow: condylar cartilage defect. Scale bar: 500μm.
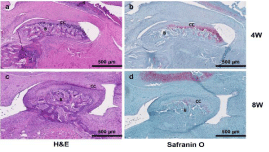
Figure 5: Histological analysis. H&E and Safranin O staining of the
experimental group (H-group) at 4- and 8-week time points. B: Bone; CC:
Condylar Cartilage. Scale bar: 500μm.
Discussion
In the present study, following the creation of a defect model (CD-group), the hydrogel-biosilica compound seeded with MSCs demonstrated effectiveness in cartilage regeneration (H-group) after 4 weeks. The CD-group showed pathological signs such as degenerative changes including the condyle and the glenoid fossa and increase in disorganized immature chondrocytes, whereas the H-group revealed an intact articular surface on the condyle and glenoid fossa, in addition to maturation and even distribution of chondrocytes along the condyle. An interesting finding was comparing the sham slide to the H slide; both showed intact fibrocartilage with no degenerative changes, but the H slide demonstrated a more mature and organized distribution of chondrocytes. It is possible that the surgical intervention triggered an inflammatory process in the sham group, while the hydrogel-biosilica compound seeded with MSCs helped to modulate that process in the H-group.
To the author’s knowledge, this mouse model demonstrates the 1st surgical disease as well as orthotopic regeneration model in mice, and rodents in general. As reported by Helgeland et al. (2018) [16] mice were solely employed as ectopic preclinical regeneration models, where the tissue engineered therapeutic components had been placed in the mice’s dorsum. Implantation of scaffolds with or without cells ranged from tissue engineered disc and mandibular condyles to explanted defected TMJ structures from other animals [3,17]. On the other hand, orthotopic models for condylar regeneration included either the rabbit, the goat or the sheep. To date, in respect to defect models, mice and rats had been mainly used for chemically induced TMJ injury and degeneration, whereas larger animals for surgical approaches, such as condylar fracture and disc perforation or displacement [1,18]. In this study, the initial challenge was the surgical access in a small animal, but the surgical technique was eventually reproducible as described by Hakim et al. (2020) [15]. The selection of the mouse as preclinical model represents an initial step that demonstrated potential efficacy of the MSCs-hydrogel-biosilica compound, at a low cost. A larger animal model will be another step towards clinical implementation, and the larger the animal, the more likely that the process can be undertaken arthroscopically.
In vivo studies with fibrocartilage orthotopic regeneration in the literature include larger animals compared to rodents - rabbits, goats and sheep. No studies were encountered, employing similar approach or biomaterials to the present experimental model.
Biomaterial scaffolds seeded with cells or enriched with growth factors demonstrate superior regenerative capacity compared to scaffolds alone [16]. Dormer et al. (2011) [19], described a novel gradient-based scaffold made of poly(D,L-lactic-co-glycolic acid) (PLGA) microspheres with transforming growth factor (TGF-β1)- loaded microspheres and Bone Morphogenetic Protein (BMP-2)- loaded microspheres. It enhanced the fibrocartilage regeneration, resulting in an even cartilaginous surface on the defected condyle of a rabbit model. Similarly, FGF-2 led to successful condylar cartilage creation, in a rabbit defect model [20]. However, one of the latest in vivo studies, did not employ cells nor growth factors. They created bilaterally osteochondral defects on the TMJs of a goat model and tested the efficiency of gelatin and synthetic sponges in condylar fibrocartilage regeneration. Despite the inadequate bone growth, it was concluded that the biomaterials have the potential for cartilaginous development [21]. Furthermore, Coskun et al. (2018) [22] recently attempted to promote cartilage and subchondral bone formation by injecting Platelet-Rich Plasma (PRP) via arthrocentesis. The model used was a rabbit, and TMJ osteoarthritis was chemically induced. Their results were optimistic, although the difference was not statistically significant.
Over the past decade, advances in the field of nanotechnology, tissue engineering and stem cell therapies have led to the development of less invasive and alternative treatments for diseased joint tissues [23,24]. Cell-based therapies involving expansion and transplantation of stem cells combined with a carrier substrate, as functional nanoparticles (i.e. biosilica), has been shown regenerative capabilities to repair diseased tissues [25-27]. The hydrogel-biosilica scaffold used in the present study holds the potential of being a nano-carrier system that might have the capability of modulating cell recruitment and serving as a regenerative matrix for cartilage repair.
Preclinical studies [24,28] and clinical trials [29] have demonstrated the efficacy of autologous or allogeneic MSCs in cartilage repair [30]. The MSCs employed in the present experimental approach derived from mice TMJ condyles. In a previous study, the authors have shown the existence of a CD105+ MSC subgroup in the TMJ fibrocartilage [12]. They were characterized for their capacity of differentiating into several cell types and showed multi-differentiation ability and clonogenicity, especially ability of chondrogenic induction [12], important for cartilage repair.
The results of this study were limited by the numbers of samples available for histological assessment, although the limited samples demonstrated cartilage degeneration of the CD-group and the potential regeneration of the H-group using MSCs seeded in a hydrogel-biosilica scaffold. Researchers using this mouse model for additional studies should consider refining the methods of obtaining histological slides prior to starting studies since the process is challenging due to the small size of the animal. Immunohistochemical analysis detecting collagen type I or II, would strengthen the validity of the results and could confirm the condylar cartilage repair/ regeneration. In the present study, it cannot be determined to what extent the hydrogel-biosilica compound remained in the TMJ throughout the 4- and 8-week timepoints. The low cost of this pilot study is a step towards additional research testing the regenerative potential of the MSC-hydrogel-biosilica compound in osteochondral TMJ defects in a larger animal to evaluate the mechanisms underlying the healing process of osteochondral defects. Future studies could also use the contralateral TMJ as healthy control, as it could prevent the unilateral overload of the operated joint.
Conclusion
In conclusion, within the limitations of this study, the MSCs+hydrogel+biosilica compound may represent an experimental therapeutic approach for TMJ condylar cartilage regeneration. More studies are warranted to test and support these findings.
Acknowledgment
This study was funded in part by the following grants: MGHDepartment of Oral and Maxillofacial Surgery Education Research Fund (Boston, MA, USA), Jean Foundation (NH), and MGH-Walter C. Guralnick Fund (Haseotes-Bentas Foundation, Boston, MA, USA).
Ethical Approval
Institutional Animal Care and Use Committee (IACUC) at the Massachusetts General Hospital (Protocol # 2017N000086).
Authors Agreement
All authors have viewed and approved the manuscript for submission.
References
- Almarza AJ, Hagandora CK, Henderson SE. Animal models of temporomandibular joint disorders: implications for tissue engineering approaches. Ann Biomed Eng. 2011; 39: 2479-2490.
- List T, Jensen RH. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia. 2017; 37: 692-704.
- Schinke B, Muhammad H, Bode C, et al. A discoidin domain receptor 1 knock out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2014; 71: 1081-1096.
- Wang F, Hu Y, He D, et al. Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice. J Craniomaxillofac Surg. 2017; 45: 855- 861.
- Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989; 115: 469- 477.
- Horton WE Jr, Bennion P, Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact. 2006; 6: 379-381.
- Xu L, Polur I, Lim C, et al. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthr Cartilage. 2009; 17: 917-922.
- Embree MC, Iwaoka GM, Kong D, et al. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthr Cartilage. 2015; 23: 629-639.
- Zhao Y, Liu P, Chen Q, et al. Development process of traumatic heterotopic ossification of the temporomandibular joint in mice. J Craniomaxillofac Surg. 2018; 47: 1155-1161.
- Dai J, Ouyang N, Zhu X, et al. Injured condylar cartilage leads to traumatic temporomandibular joint ankylosis. J Craniomaxillofac Surg. 2015; 44: 294- 300.
- Ouyang N, Zhu X, Li H, et al. Effects of a single condylar neck fracture without condylar cartilage injury on traumatic heterotopic ossification around the temporomandibular joint in mice. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 125: 120-125.
- Thamm JR, Jounaidi Y, Mueller ML, et al. Temporomandibular joint cartilage contains CD105 positive mouse mesenchymal stem/progenitor cells with increased chondrogenic potential. 2021.
- Goren R, Baykara T, Marsoglu M. A study on the purification of diatomite in hydrochloric acid. Scand J Metall. 2002; 31: 115-119.
- Ruggiero I, Terracciano M, Martucci NM, et al. Diatomite silica nanoparticles for drug delivery. Nanoscale Res Lett. 2014; 9: 329.
- Hakim MA, Guastaldi FP, Liapaki A, et al. In vivo investigation of temporomandibular joint regeneration: development of a mouse model. Int J Oral Maxillofac Surg. 2020; 49: 940-944.
- Helgeland E, Shanbhag S, Pedersen TO, et al. Scaffold-based temporomandibular joint tissue regeneration in experimental animal models: A systematic review. Tissue Eng Part B Rev. 2018; 24: 300-316.
- Cheng B, Tu T, Shi X, et al. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res Ther. 2019; 10: 298.
- Almarza AJ, Brown BN, Arzi B, et al. Preclinical animal models for temporomandibular joint tissue engineering. Tissue Eng Part B Rev. 2018; 24: 171-178.
- Dormer NH, Busaidy K, Berkland CJ, et al. Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. J Oral Maxillofac Surg. 2011; 69: e50-57.
- Takafuji H, Suzuki T, Okubo Y, et al. Regeneration of articular cartilage defects in the temporomandibular joint of rabbits by fibroblast growth factor-2: a pilot study. Int J Oral Maxillofac Surg. 2007; 36: 934-937.
- Chin AR, Gao J, Wang Y, et al. Regenerative Potential of Various Soft Polymeric Scaffolds in the Temporomandibular Joint Condyle. J Oral Maxillofac Surg. 2018; 76: 2019-2026.
- Coskun U, Candirli C, Kerimoglu G, et al. Effect of platelet-rich plasma on temporomandibular joint cartilage wound healing: Experimental study in rabbits. J Craniomaxillofac Surg. 2019; 47: 357-364.
- Aryaei A, Vapniarsky N, Hu JC, et al. Recent tissue engineering advances for the treatment of temporomandibular joint disorders. Curr Osteoporos Rep. 2016; 14: 269-279.
- Salash JR, Hossameldin RH, Almarza AJ, et al. Potential indications for tissue engineering in temporomandibular joint surgery. J Oral Maxillofac Surg. 2016; 74: 705-711.
- Urech DM, Feige U, Ewert S, et al. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNFa single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann Rheum Dis. 2010; 69: 443-449.
- Brady MA, Sivananthan S, Mudera V, et al. The primordium of a biological joint replacement: coupling of two stem cell pathways in biphasic ultrarapid compressed gel niches. J Cranio Maxill Surg. 2011; 39: 380-386.
- Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013; 9: 584.
- Xia T, Yu F, Zhang K, et al. The effectiveness of allogeneic mesenchymal stem cells therapy for knee osteoarthritis in pigs. Ann Transl Med. 2018; 6: 404.
- Iijima H, Isho T, Kuroki H, et al. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: A meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018; 3: 15.
- Lee YH, Park HK, Auh QS, et al. Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis. Int J Mol Sci. 2020; 21: E1541.