
Research Article
J Dent & Oral Disord. 2021; 7(3): 1163.
Matrix Metalloproteinase-2 Gene Polymorphisms Decrease Chances of Muscle TMD Development in Brazilian Population
Cordeiro PCF1, Bonato LL2, Quinelato V1*, Santos TAB2, Vieira AR3, Granjeiro JM4, Tesch R5, Casado PL6, Calasans-Maia JA1
1Orthodontics Department, Universidade Federal Fluminense, Dentistry School, Brazil
2School of Dentistry, Universidade Federal Fluminense, Brazil
3Departments of Oral Biology and Pediatric Dentistry, University of Pittsburgh, School of Dental Medicine, Pittsburgh, USA
4Laboratory of Clinical Research in Dentistry, Universidade Federal Fluminense, Dentistry School, Brazil
5School of Medicine of Petropolis, Brazil
6Universidade Federal Fluminense, Brazil
*Corresponding author: Valquíria Quinelato, Orthodontics Department, School of Dentistry, Universidade Federal Fluminense (Mario Santos Braga St, 30/214 - Center – Niterói-RJ, Brazil
Received: April 06, 2021; Accepted: May 06, 2021; Published: May 13, 2021
Abstract
Introduction: Muscular Temporomandibular disorders or masticatory disorders are characterized by orofacial pain and functional limitations associated with oral dysfunctions, emotional changes and/or genetic factors. Matrix Metalloproteinases (MMPs) are proteolytic enzymes that constitute the extracellular matrix and play an important role in the skeletal muscle adaptation.
Objectives: To evaluate the association between polymorphisms in the Matrix Metaloprotease-2 (MMP2) gene and the presence of muscular disorders.
Methods: RDC/TMD questionnaire was applied for clinical diagnosis of Temporomandibular Disorders (TMD) in the study sample and three diagnosis groups were formed: control group (n=154), muscular TMD (n=122) and joint TMD (n=49). Genomic DNA was obtained from saliva samples and six single nucleotide polymorphisms in the MMP2 gene were selected. Results: A tendency of association between the presence of the CT genotype (rs243865) and the absence of muscular TMD was observed when compared to the control group (p=0.05). There was a significant prevalence of the polymorphic CT+TT (rs243865) genotypes in the control group (p=0.04) compared to the muscular TMD group (p=0.05). Confirming these results, TCCACC MMP2 haplotype showed higher association (p=0.01) with protection against muscular TMD.
Conclusion: Polymorphism in the MMP2 gene (rs243865) is related to protection against muscular TMD.
Keywords: Temporomandibular joint dysfunction syndrome; Polymorphism genetic; Matrix Metalloproteinase-2
Introduction
Temporomandibular Disorders (TMD) collectively comprise a set of clinical conditions associated with pain and/or dysfunction located in the masticatory musculature and/or Temporomandibular Joints (TMJ) [1] with muscular disorders being the single most prevalent diagnostic group [2]. The diagnosis of muscular TMD is based on the presence of clinical signs and symptoms standardized in the form of criteria [3]. However, these patients do not evolve uniformly, many presenting disproportionate pain in response to different types of stimuli [1,2,4], as well as comorbid, regional, or systemic pain [5]. In addition, in some patients, pain complaints persist even after resolution of the initial injury [2,6].
It is currently known that chronic muscular pain, including masticatory pain, is influenced by neuronal, central, and peripheral sensitization phenomena [7], often influenced by predisposing genetic factors. The genetic influence involved in the pathophysiology of TMD has been previously demonstrated in different studies related to different genes - ESR1, ESRRB, RANK, RANKL, and OPG [6,8]. In addition to pain thresholds, the genetic profile of each patient also proved to be able to regulate muscle regenerative capacity [2].
Adult skeletal muscle tissue, including masticatory muscle, has high regenerative capacity in response to acute or chronic injuries. As skeletal muscle fibers are in their terminal stage of differentiation and therefore unable to participate in tissue repair, this response is largely attributed to a small, distinct population of myogenic progenitor cells resident in adult skeletal muscle, called Satellite Cells (SC) [9,10]. SC can be activated following chemical and mechanical stimuli, migrating to the injury site and orchestrating muscle regeneration [9]. Mature muscle fibers, in turn, produce and are involved by an Extracellular Matrix (ECM), which also acts in tissue repair through the release of specific biochemical mediators [11,12], mainly those called Matrix Metalloproteinases (MMPs).
MMPs constitute a broad heterogeneous family of enzymes responsible for breaking peptide bonds between protein amino acids [9,12]. In muscle tissue, MMP-2 and MMP-9, produced by myoblasts, fibroblasts, and endothelial cells [11,13] are prominent, creating a favorable environment for muscle repair [14]. MMPs are responsible for ECM renewal, increasing its chemotactic potential [10,15]. They are, for example, responsible for the cleavage and activation of Hepatocyte Growth Factor (HGF) [9], enabling it to bind with SC membrane receptors, such as c-Met, activating them and thus, inducing tissue repair by cell proliferation and differentiation [15]. In addition, MMP-2 is even involved in angiogenic processes after mechanical stimuli, capable of generating myofibrillar hypertrophic effects (Figure 1) [16].
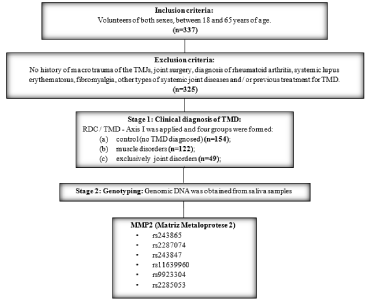
Figure 1: Flowchart of the study steps.
The gene encoding MMP-2 is located at the 16q13-q21 locus, with the C to T allelic variation, located at nucleotide -1306, being related to low transcription activity of this gene [17]. Thus, predisposition to pathologies occurs especially when variants of the MMP2 gene are associated with increased MMP-2 concentration, including chronic pain often comorbid with muscular TMD, such as migraine with aura [18,19].
Therefore, based on the role of these proteins in the pathophysiology of muscle lesions, as well as the natural regenerative processes following them, it was hypothesized that variants of the MMP2 gene could partially explain the variability of physiological tolerance and functional adaptation of the masticatory musculature, contributing to the onset and progression of the different manifestations of muscular TMD. Thus, the present study aimed to evaluate the possible association between genetic polymorphisms in regions rs243865, rs2287074, rs243847, rs11639960, rs9923304, and rs2285053 of the MMP2 gene and the frequency of occurrence of muscular TMD.
Methods
The study design is observational and cross-sectional, approved by the Research Ethics Committee of the Hospital Universitário Antônio Pedro/Fluminense Federal University, on September 25, 2016, under opinion number 1.744.837. Free and informed consent forms were received and signed by the participants before the research carried out. In developing its design, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [20] recommendations were follow ed.
The study included individuals attended at the Fluminense Federal University (who were patients, or those accompanying them, seeking attention at the institution) between 18 and 65 years of age. Individuals were randomly selected over a period of two years. Exclusion criteria were: history of macro trauma in the TMJ region, joint surgery, diagnosis of rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia, other types of systemic joint diseases, and/or previous treatment for TMD. The methodology of the study was divided into two stages:
1st stage - clinical diagnosis of TMD
All the participants in the study were clinically examined by the same evaluator using the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) - Axis I (Schiffman et al. 2014), validated for the physical diagnosis of TMD. It allowed classification of participants in any of the following diagnostic subgroups: 0) Absence of TMD; 1) Painful muscle disorders; 2) Changes in the position of the articular disc; and 3) Painful and/or degenerative TMJ conditions. This process works in a non-mutually exclusive manner, allowing each participant to belong to more than one diagnostic sub-group.
The joint diagnoses (subgroups 2 and 3) were grouped together, forming a single “joint disorders” group. Thus, three groups were formed: a) Control (absence of TMD diagnosis); b) With muscle disorders; c) With joint disorders exclusively. This tool was applied to all the research participants by a single trained examiner (author L.L.B.).
2nd stage - Genotyping
Genomic DNA was obtained from saliva samples from all participants, as previously described [21]. The concentration and purity of the DNA were analyzed using the NanoDrop® spectrophotometer (Thermo Scientific, Wilmington, DE, USA). All samples had to have an A260nm/A280nm ratio greater than 1.9.
Six single nucleotide polymorphisms in the MMP2 gene (rs243865, rs2287074, rs243847, rs11639960, rs9923304, rs2285053) were selected, considering binding disequilibrium ratios and gene structure (Figure 1). These SNPs were previously identified and included in the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/SNP/), with the lowest acceptable allele frequency being >0.12. All procedures followed the STREGA recommendations [21], just as in previous studies on the influence of different genes on the pathophysiology of TMD - ESR1, ESRRB, RANK, RANKL, and OPG [6,8].
Data processing and statistical analysis were done using the STATA 12.0 program (StataCorp, College Station, TX, USA). The sample size included the spontaneous demand of patients and accompanying persons at the Fluminense Federal University, respecting the inclusion criteria. This calculation was designed to detect a risk factor that attained 40% of the sample, with a 5% alpha and 90% power. To verify normal distribution of the numerical variables, the Shapiro-Wilk test was used, followed by analysis of variance with the Student’s t-test and the Mann-Whitney test, in the cases of normal and non-normal distribution, respectively. To evaluate the significance of the nominal variables between the groups, the chi-square test was used.
Differences in the frequency of genotypes and alleles between groups were analyzed using the chi-square test after assembly for the Hardy-Weinberg equilibrium. Statistical differences between groups were calculated using the chi-square test or Fisher’s exact test. To calculate linkage disequilibrium and haplotypes, the ARLEQUIN software program was used (v.20; http://anthro.unige.ch/arlequin). Values of p≤0.05 were considered statistically significant, and the risks associated with individual alleles and genotypes were calculated as the odds ratio (OR) with a 95% Confidence Interval (CI).
Results
Characterization of the sample
From a total of 337 research participants evaluated over the two-year period, 325 were included in the study. One hundred and twenty-two (37.5%) had muscular TMD, 49 (15.1%) had only joint TMD, composing a control group with pain, and 154 (47.4%) had no type of painful disorder, representing the pain-free control group.
The muscular TMD group consisted of 85 (70%) women and 37 (30%) men, and the control group with joint TMD was composed of 32 (65.3%) women and 17 (34.7%) men. While in the TMD-pain-free control group, 94 (61%) were women and 60 (39%) were men. The overall mean age for all groups was 45.1 ± 12.3 years. There was no significant difference between gender and risk of developing muscle (p=0.17) or joint (p=0.71) disorders.
Analysis of the genetic association
The characteristics of the six polymorphisms studied in the MMP2 gene are presented in Table 1. A statistically significant higher prevalence of the polymorphic genotypes (CT + TT) could be observed in the individuals in the pain-free control group (p=0.04) (Table 2).
Gene symbol
Gene name
SNP
Chromosome
Base pair position*
SNP type
Base changea
MFAb
Major
Minor
MMP2
Matrix metalloprotease 2
rs243865
16
55477894
Upstream variant
C
T
0.13
rs2287074
55493201
Synonymous codon
A
C
32
rs243847
55490086
Intragenic
C
T
0.34
rs11639960
55499358
Intragenic
A
G
0.23
rs9923304
55496389
Intragenic
C
T
0.31
rs2285053
55478465
Upstream variant
C
T
0.15
SNP: Single Nucleotide Polymorphism. aBase change according to Applied Biosystems. bMAF: Minor Allele Frequency according to GenBank.
Table 1: Characteristics of the polymorphisms studied in the MMP2 gene.
Gene
SNP
Genotypes
Control (n=154)
Muscular (n=122)
Articular (n=49)
P-value (OR; CI)
Control x Muscular
Muscular x Articular
MMP2
rs243865
CC-CT-TT
91-53-2
88-27-3
32-17-0
0.05
0.12
CT+TT
55
30
17
0.04 (0.5 (0.3-0.9)
0.3 (1.5 (0.7-3.2)
C
235
203
81
0.11 (1.4 (0.9-2.3)
0.5 (0.7 (0.4-1.4)
T
57
33
17
rs2287074
AA-AC-CC
15-85-47
15-65-39
6-31-12
0.79
0.53
AC+CC
132
104
43
0.67 (0.7 (0.3-1.6)
0.8 (1.0 (0.3-2.8)
A
115
98
43
0.77 (1.0 (0.7-1.5)
0.6 (1.1 (0.7-1.8)
C
179
143
55
rs243847
CC-CT-TT
16-81-52
13-54-52
4/23/2021
0.3
0.87
CT+TT
133
106
44
0.88 (0.9 (0.4-2.1)
0.4 (1.3 (0.4-4.3)
C
113
80
31
0.34 (0.8 (0.5-1.1)
0.9 (0.9 (0.5-1.5)
T
185
158
65
rs11639960
AA-AG-GG
73-64-5
65-45-8
22-23-2
0.32
0.42
AG+GG
69
53
25
0.63 (0.8 (0.5-1.4)
0.4 (1.3 (0.7-2.7)
A
210
175
67
0.51 (1.0 (0.6-1.4)
0.6 (0.8 (0.5-1.4)
G
74
61
27
rs9923304
CC-CT-TT
56-79-14
42-62-14
14-29-6
0.8
0.67
CT+TT
93
76
35
0.84 (1.0 (0.6-1.7)
0.4 (1.3 (0.6-2.8)
C
191
146
57
0.66 (0.9 (0.6-1.2)
0.6 (0.8 (0.5-1.3)
T
107
90
41
rs2285053
CC-CT-TT
108-26-2
83-26-0
34-12-0
0.22
0.77
CT+TT
28
26
12
0.64 (1.2 (0.6-2.2)
0.9 (1.1 0.5-2.4)
C
242
192
80
0.86 (0.91 (0.5-1.5)
0.9 (0.9 (0.4-1.8)
T
30
26
12
Table 2: Distribution of genotypes and alleles of the MMP2 gene.
Haplotype analysis showed a significant association between the TCCACC haplotype and protection against muscular TMD (p=0.01), (Table 3). The results (p=0.05) for the TCCACC haplotype, showing association with muscular TMD protection.
Gene
Haplotypes
P-value
Control (n=154)
Muscular (n=122)
Articular (n=49)
Control x Muscular
Muscular x Articular
MMP2
CCCACC
0.28
0.28
0.27
-
-
CCTACC
0.17
0.19
0.16
0.7
0.91
CATGTC
0.12
0.13
0.18
0.65
0.32
TCCACC
0.06
0.006
0.04
0.01
0.06
Table 3: Haplotypes.
Discussion
Masticatory muscles differ from other skeletal muscles due to greater functional requirements for executing various specific motor activities, such as chewing, speech, and swallowing [22,23]. Thus, they are able to precisely control mandibular positioning and, simultaneously, alter the levels of force required for each activity [23]. However, the exacerbated mechanical induction in parafunctional situations perturbs the local blood flow, generating hypoxia and/or ischemia, and thus, neuroactive and inflammatory biomarkers signal inflammatory and regenerative processes. In situations of imbalance of ECM components, pain signals are generated, characterizing the development of muscular TMD [24].
In order to understand the mechanisms involved in the masticatory muscular process, the expression of MMP-2 and histological characteristics of the masseter muscle after unilateral tooth extraction were analyzed. The authors concluded that the intervention induced mechanical loading and, consequently, remodeling of muscle fibers, evidenced by the high levels of MMP-2 on the intervention side [25]. Since the physiological mechanisms related to muscle adjustments cause qualitative and quantitative changes in the structure of the masticatory muscles [26], as well as morphofunctional changes, such as osteoporosis of the jaw, there is a greater need for additional recruitment of muscle fibers in mastication, generating stress and tissue fatigue [27].
Occlusal asymmetries, such as those related to malocclusion with unilateral posterior crossbite, may also alter mandibular kinematics with reverse chewing cycles and change in masseter muscle coordination, demonstrating less activation or even muscle atrophy on the affected side. In contrast, the contralateral side exhibits greater remodeling of the ECM and proliferation of SC possibly related to greater mechanical overload [28]. Similarly, other authors have observed positive regulation of MMP-2 in reperfusion injury after ischemia in the skeletal muscle of rats, therefore concluding that the inhibition of enzyme activity presents therapeutic potential in reducing the severity of these lesions [29].
The remodeling of the craniofacial musculature with genetic predisposition can also be observed in individuals with altered facial forms. Tippett et al. performed biopsies on 20 volunteers (10 with hyperdivergent facial type and 10 with facial divergence) to evaluate the proteolytic activity of the MMP2 and MMP9 genes. The results demonstrated individual variation in the expression and suggested that there is a relationship between the reduction of MMP-2 activity, the vertical facial pattern, and the consequent needs for adaptive muscle function [30].
In this context, MMP-2 activity, widely described in muscle repair processes, may be aided or impaired by alterations of this coding gene. The first region of the MMP2 gene evaluated in the present study (rs243865) had already been investigated in previous studies, demonstrating association with several pathologies [31-33]. In these studies, the C (Cytosine) to T (Thymine) allelic variation (rs243865) was associated with the lower gene expression of MMP- 2. In contrast, the CC genotype (cytosine-cytosine) proved to be associated with higher MMP-2 expression [33]. In the present study, the polymorphisms were more prevalent in individuals free of masticatory muscular pain, suggesting a protective relationship related to the equilibrium of the activity of these enzymes.
However, longitudinal studies need to be conducted, as well as other genetic studies, considering specific miRNA analysis and the level of gene expression and tissue proteins in individuals diagnosed with TMD. The lack of hypotheses or specific analyses of the MMP2 gene in individuals with TMD does not imply the absence of association, further emphasizing the importance of data analysis in larger samples and in the Brazilian population, if possible, for a better discussion of the results presented.
The present study made it possible to trace the genetic profile of individuals with muscular TMD by means of polymorphic regions. Based on results, it is suggested that there is genetic influence in the expression and/or function of MMP-2 and, consequently, in SC activation, implying an increase in the risk of developing muscular TMD. Therefore, the importance and uniqueness of the association studied here is highlighted, which opens the way to a better understanding of different genetic characteristics related to chronic orofacial pain, as well as to the development of personalized therapeutic behaviors.
Conclusion
Polymorphism in the MMP2 gene (rs243865) is related to protection against muscular TMD.
References
- Okeson JP, de Leeuw R. Differential Diagnosis of Temporomandibular Disorders and Other Orofacial Pain Disorders. Dent Clin North Am. 2011; 55: 105-120.
- Manfredini D, Favero L, Cocilovo F, Monici M, Guarda-Nardini L. A comparison trial between three treatment modalities for the management of myofascial pain of jaw muscles: A preliminary study. Cranio - J Craniomandib Pract. 2018; 36: 327-331.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J-P, et al, International RDC/TMD Consortium Network, International association for Dental Research, Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J oral facial pain headache. 2014; 28: 6-27.
- Visscher CM, Lobbezoo F. TMD pain is partly heritable. A systematic review of family studies and genetic association studies. J Oral Rehabil. 2015; 42: 386-399.
- Bonato LL, Quinelato V, De Felipe Cordeiro PC, De Sousa EB, Tesch R, Casado PL. Association between temporomandibular disorders and pain in other regions of the body. J Oral Rehabil. 2017; 44: 9-15.
- Bonato LL, Quinelato V, Pinheiro ADR, Amaral MVG, De Souza FN, Lobo JC, et al. ESRRB polymorphisms are associated with comorbidity of temporomandibular disorders and rotator cuff disease. Int J Oral Maxillofac Surg. 2016; 45: 323-331.
- Cairns BE. Pathophysiology of TMD pain--basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil. 2010; 37: 391-410.
- Bonato LL, Quinelato V, Borojevic R, Vieira AR, Modesto A, Granjeiro JM, Tesch R, et al. Haplotypes of the RANK and OPG genes are associated with chronic arthralgia in individuals with and without temporomandibular disorders. Int J Oral Maxillofac Surg. 2017; 46: 1121-1129.
- Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adhes Migr. 2009; 3: 337-341.
- González MN, de Mello W, Butler-Browne GS, Silva-Barbosa SD, Mouly V, Savino W, Riederer I. HGF potentiates extracellular matrix-driven migration of human myoblasts: Involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet Muscle. 2017; 7: 1-13.
- Alameddine HS. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol Dis. 2012; 48: 508-518.
- Tunc-Ata M, Mergen-Dalyanoglu M, Turgut S, Turgut G. Effect of acute and chronic exercise on plasma matrix metalloproteinase and total antioxidant levels. J Exerc Rehabil. 2017; 13: 508-513.
- Nowak E, Gawor M, Ciemerych MA, Zimowska M. Silencing of gelatinase expression delays myoblast differentiation in vitro. Cell Biol Int. 2018; 42: 373-382.
- Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol. 2008; 52: 307-314.
- Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, et al. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol. 2008; 40: 2183-2191.
- Ross MD, Wekesa AL, Phelan JP, Harrison M. Resistance exercise increases endothelial progenitor cells and angiogenic factors. Med Sci Sports Exerc. 2014; 46: 16-23.
- Price SJ, Greaves DR, Watkins H. Identification of Novel, Functional Genetic Variants in the Human Matrix Metalloproteinase-2 Gene. J Biol Chem. 2002; 276: 7549-7558.
- Jacob-Ferreira ALB, Lacchini R, Gerlach RF, Passos CJS, Barbosa F, Tanus- Santos JE. A common matrix metalloproteinase (MMP)-2 polymorphism affects plasma MMP-2 levels in subjects environmentally exposed to mercury. Sci Total Environ. 2011; 409: 4242-4246.
- Gonçalves FM, Martins-Oliveira A, Lacchini R, Belo VA, Speciali JG, Dach F, et al. Matrix metalloproteinase (MMP)-2 gene polymorphisms affect circulating MMP-2 levels in patients with migraine with aura. Gene. 2013; 512: 35-40.
- Elm E Von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. 2008; 61: 344-349.
- Küchler EC, Tannure PN, Falagan-Lotsch P, Lopes TS, Granjeiro JM, Maria LMF, et al. Buccal cell’s DNA extraction to obtain high quality human genomic DNA suitable for polymorphism. J Appl Oral Sci. 2012; 20: 467-471.
- Korfage JAM, Kwee KE, Everts V, Langenbach GEJ. Myosin Heavy Chain Expression Can Vary Over the Length of Jaw and Leg Muscles. Cells Tissues Organs. 2016; 201: 130-137.
- Aare S, Ochala J, Norman HS, Radell P, Eriksson LI, Göransson H, et al. Mechanisms underlying the sparing of masticatory versus limb muscle function in an experimental critical illness model. Physiol Genomics. 2011; 43: 1334-1350.
- Guerra Cde S, Carla Lara Pereira Y, Issa JP, et al. Histological, histochemical, and protein changes after induced malocclusion by occlusion alteration of Wistar rats. Biomed Res Int. 2014; 2014: 563463.
- Maluly M, Andersen ML, Garbuio S, Bittencourt L, Siqueira JTT De, Tufik S. Polysomnographic Study of the Prevalence of Sleep Bruxism in a Population Sample. J Dent Res. 2013; 92: 97-103.
- Vasconcelos PB, Palinkas M, Sousa LG De, Regalo SCH, Santos CM, Rossi M De, et al. The influence of maxillary and mandibular osteoporosis on maximal bite force and thickness of masticatory muscles. Acta Odontol Latioamericana. 2015; 28: 22-27.
- Cutroneo G, Vermiglio G, Centofanti A, Rizzo G, Runci M, Favaloro A, et al. Morphofunctional compensation of masseter muscles in unilateral posterior crossbite patients. Eur J Histochem. 2016; 60: 2605.
- Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA. Upregulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur J Vasc Endovasc Surg. 2002; 23: 260-269.
- Tippett HL, Dodgson LK, Hunt NP, Lewis MP. Indices of extracellular matrix turnover in human masseter muscles as markers of craniofacial form--a preliminary study. Eur J Orthod. 2008; 30: 217-225.
- Tippett HL, Dodgson LK, Hunt NP, Lewis MP. Indices of extracellular matrix turnover in human masseter muscles as markers of craniofacial form-a preliminary study. Eur J Orthod. 2008; 30: 217-225.
- Lin CM, Zeng YL, Xiao M, Mei XQ, Shen LY, Guo MX, et al. The relationship between MMP-2-1306C>T and MMP-9-1562C>T polymorphisms and the risk and prognosis of T-cell acute lymphoblastic leukemia in a Chinese population: A case-control study. Cell Physiol Biochem. 2017; 42: 1458-1468.
- Liutkeviciene R, Lesauskaite V, Sinkunaite-Marsalkiene G, Simonyte S, Zemaitiene R, Kriauciuniene L, et al. MMP-2 Rs24386 (C-->T) gene polymorphism and the phenotype of age-related macular degeneration. Int J Ophthalmol. 2017; 10: 1349-1353.