
Research Article
J Dent & Oral Disord. 2023; 9(1): 1177.
Periodontal Status and Gestational Diabetes Mellitus: A Case-Control Study
Estévez-Llorens R1*, Menéndez-Nieto I1, Baquero-Ruiz de la Hermosa MC2, Marcos-Puig B3 and Perales-Marín A3,4
1Department of Dentistry, Faculty of Health Sciences.Universidad Europea de Valencia, Spain
2Oral and Maxillofacial Surgery Service, Hospital U.P La Fe, Valencia, Spain
3Obstetrics and Gynecology Service, Hospital U.P La Fe, Valencia, Spain
4Pediatrics, Department of Obstetrics and Gynecology, Universidad de Valencia, Spain
*Corresponding author: Raquel Estévez LlorensUniversidad Europea de Valencia, Faculty of Health Sciences, Department of Dentistry, Paseo de la Alameda, 7, 46010, Valencia, Spain
Received: December 14, 2022; Accepted: January 20, 2023; Published: January 27, 2023
Abstract
Background: Studies linking Periodontal Disease (PD) and Gestational Diabetes Mellitus (GDM) are not in agreement. Our main objective is to evaluate the possible association between PD and GDM.
Methods: 222 pregnant women participated, 111 with GDM, from Hospital La Fe de Valencia. Periodontal examination was performed, assessing the following parameters: number of teeth, plaque index, bleeding on probing, Pocket Probing Depth (PPD) and Clinical Attachment Level (CAL).
Results: The GDM had a higher value of PPD (p = 0.001) and CAL (p = 0.013). 75.7% of the patients with GDM had gingival inflammation compared to 56.8% of the non-diabetic patients. Periodontitis was more prevalent in patients with GDM (p < 0.05).
Conclusions: The results suggest that pregnant women with gestational diabetes associate more periodontal disease than those without such gestational disease. In our sample, the risk of GDM can be estimated from periodontitis, age and educational level. However, no relation of statistical significance has been found between a worse periodontal condition and the need of insulin in the treatment of GDM or having more adverse pregnancy outcomes in the GMD.
Keywords: Gestational diabetes mellitus; Periodontal disease; Gingivitis; Periodontitis; Pregnancy; Insulin
Introduction
Periodontal Disease (PD) includes infectious pathologies that affect the supporting tissues of the tooth. Gingivitis only affects the gums and is a reversible inflammatory process, while periodontitis is a multifactorial bacterial infection that, in addition to gingival inflammation, causes irreversible destruction of the supporting structures of the tooth [1,2]. Periodontal diseases affect a large part of the population worldwide, with advanced periodontitis being the sixth most prevalent disease on the planet [3].
Gestational Diabetes Mellitus (GDM) is a glucose intolerance detected for the first time during pregnancy, with a prevalence of 10-25% of pregnancies, depending on the population studied and the diagnostic criteria used. It is the most common medical complication of pregnancy [4]. GDM is associated with preeclampsia, abortion, premature births and type 2 diabetes (DM2) in the future [5,6].
In pregnant women, periodontitis has been associated with premature births, low birth weight newborns, and preeclampsia [7-11]. There are different studies suggesting the relationship between periodontitis and GDM. However, the results are contradictory [12,13]. Establishing a relationship between poor periodontal health and GDM would help reduce the incidence of GDM and its associated adverse outcomes.
Our main goal is to study the possible relationship between GDM and PD. Secondary objectives are to analyze if patients with GDM and in worse periodontal condition have worse blood glucose control and more adverse pregnancy outcomes than gestational diabetic women with better periodontal health.
Material and Methods
Design
This is an observational case-control and cross-sectional study, where we compare the periodontal status of 111 pregnant women with GDM (cases) and 111 without GDM (controls), controlled in the Obstetrics and Gynecology Service of the Hospital UP La Fe de Valencia.
The inclusion criteria were: gestational age greater than 24 weeks, complete GDM screening, over 18 years of age, informed consent. The exclusion criteria were: pregestational diabetes type 1 or 2, HIV and autoimmune diseases, less than 14 teeth, having received periodontal treatment during the three months prior to the study, use of drugs, insulin or oral antidiabetics before pregnancy, consumption of corticosteroids.
Definition of Cases and Controls
The cases were pregnant women diagnosed with GDM following the diagnostic criterion recommended by the National Diabetes Data Group (NDDG) [14]. Whereas the controls had a normal oral glucose loads. Both cases and controls had the same gestational age.
Oral Health Examination
All periodontal records were made by a single dentist using a mouth mirror number 5 and PQ-W Williams periodontal probe (Hu-Friedy, Chicago, IL, USA). Each tooth was explored in 6 zones (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, distolingual). The recorded parameters were: The number of teeth that each patient had (excluding third molars). The Plaque Index (PI) is calculated as the percentage of surfaces that present plaque in relation to the total number of dental surfaces evaluated. Oral hygiene was considered acceptable when the PI was < 20%. The Bleeding Index on Probing (BOP) was the method used to assess gingival inflammation. It was considered that there was gingival inflammation when the patient presented ≥ 10% of the bleeding locations [15]. Pocket Probing Depth (PPD) was measured as the distance from the gingival margin to the bottom of the periodontal pocket. And Clinical Attachment Level (CAL) was calculated from the measurements of gingival recession (distance from the cement-enamel line to the gingival margin) and probing depth. The mean attachment level and the mean probing depth were calculated.
Periodontitis was defined according to the clinical criterion of the CDC classification for population studies and the AAP (Centers for Disease Control and Prevention and the American Academy of Periodontology), also called the Page & Eke classification [16]. According to this classification, Severe Periodontitis is defined as the presence of 2 or more interproximal areas with clinical attachment loss ≥ 6 mm, not in the same tooth, and 1 or more interproximal areas with PS ≥ 5 mm. Moderate Periodontitis is described as the presence of 2 or more interproximal areas with attachment loss ≥ 4 mm, not in the same tooth or 2 or more interproximal areas with PS ≥ 5 mm not in the same tooth. Mild Periodontitis is defined as the presence of 2 or more interproximal areas with attachment loss ≥ 3mm and 2 or more interproximal areas with PS ≥ 4mm (not on the same tooth) or one area with PS ≥ 5mm. Periodontitis was considered as the presence of mild, moderate or severe periodontitis, Gingivitis as the absence of periodontitis and bleeding index on probing ≥ 10%, and Periodontal Health as the absence of periodontitis and bleeding index on probing < 10%.
To study the secondary goals, patients with GDM were separated into two groups: non-insulin dependent and insulin dependent following the ACOG (American College of Obstetricians and Gynecologists) classification according to whether or not they required insulin treatment to control the disease. These two groups were compared with the periodontal variables: PI, BOP, PPD, CAL and Periodontal criteria.
The adverse pregnancy outcomes that were analyzed were Premature Rupture of Membranes (PROM), preeclampsia, Low Birth Weight (LBW), preterm birth, macrosomia and intrauterine growth restriction (IUGR).Patients with GDM were classified into two groups according to whether they had one or more of these perinatal complications or not. These two groups were compared with the periodontal variables: PI, BOP, PPD, CAL and Periodontal criteria.
Questionnaire
The study participants completed a supervised questionnaire to collect affiliation, sociocultural, and oral and general health data.
Statistical Analysis
A descriptive and inferential statistical analysis was performed. Quantitative variables were described by means and Standard Deviations (SD). Qualitative variables were described using frequencies and relative percentages. The Kolmogorov-Smirnov test was used to determine the normality of the quantitative variables. In the case of non-normality, the non-parametric Mann-Whitney U test was applied for independent samples of two groups. In the cases of normal variables, the Student’s t test was applied for independent groups. To analyze the bivariate relationships between the qualitative variables, contingency tables were constructed and the Chi-Square test was applied, logistic regression analysis was used to characterize the independent risk factor for GDM, also ROC curve was build. Statistical analysis was performed using IBM SPSS statistics v.23 and Medcap. The significance level was set at p <0.05.
Results
On the total sample (n=222), the association of social, local, systemic factors and other factors in relation to GDM was studied (Table 1) shows the comparative analysis between cases and controls. The gestational age at which the study was performed was not different between the two groups. Pregnant women with GDM were significantly older, had higher BMI and lower educational level and relatives with diabetes history. There were no differences regarding the rest of the studied parameters.
GDM
n=111No GDM
n=111p
Age
Mean (SD)
36.63 (4.37)
34.34 (4.65)
0.000 b
Gestationalage at recruitment
Mean (SD)
32.62 (3.10)
32.21 (3.01)
0.434 c
Pregestational BMI
Mean (SD)
26.31 (6.03)
24.71 (6.08)
0.014 c
Education level
Noneorbasic
n (%)
24 (21.6)
9 (8.1)
0.013 a
Secondary
37 (33.3)
38 (34.2)
University
50 (45.1)
64 (57.7)
Employment
Employed
n (%)
78 (70.3)
91 (82.0)
0.041 a
Unemployed
33 (29.7)
20 (18.0)
Smoking
Current
n (%)
14 (12.6)
8 (7.2)
0.229 a
Previous< 10 years
27 (24.3)
22 (19.8)
Never
70 (63.1)
81 (73.0)
Frecuency of brushing
= 1 per day
n (%)
32 (28.83)
29 (26.13)
0.652 a
> 1 per day
79 (71.17)
82 (73.87)
Family 1ª grade history of diabetes
No
n (%)
69 (62.16)
84 (75.68)
0.042 a
Yes
42 (37.84)
27 (24.32)
a Χ2
b T Student
c U Mann Whitney
Table 1: Characteristics of the study population according to the case-control status.
When comparing the periodontal situation between cases and controls, as shown in (Table 2), gestational diabetic patients had a significantly lower number of teeth, 26.87±1.8 vs 27.35±1.3, p<0.05. Patients with gestational diabetes also had statistically higher average of PPD and CAL, and there were more gestational diabetics with BOP index ≥ 10% (75.7% of cases vs. 56.8% of controls, p= 0.029). The presence of periodontitis occurred in 93/111 of the gestational diabetics, while it was in 72/111 of the controls p <0.025, OR 2.36 95% CI 1.25 to 4.47. However, no statistically significant differences (p= 0.059) were found between the plaque index and GDM.
GDM
n=111No GDM
n=111p
Number of teeth
Mean nº of teethMean (SD)
26.87 (1.8)
27.35 (1.3)
<0.05c
Plaque Index
PI = 20 %
PI < 20 %n (%)
60 (54.1)
51 (45.9)
46 (41.4)
65 (58.6)
0.059a
Bleeding on Probing
BOP = 10 %
BOP < 10 %n (%)
84 (75.7)
27 (24.3)63 (56.8)
48 (43.2)0.029a
Pocket Probing Depth
Mean PPD (mm)Mean (SD)
2.33 (0.34)
2.24 (0.28)
0.001c
Clinical Attachment Level
Mean CAL (mm)Mean (SD)
2.38 (0.35)
2.29 (0.28)
0.013c
Periodontal status
Periodontal health
Gingivitis
Periodontitis*
n (%)
18 (16.2)
15 (13.5)
78 (70.3)
39 (35.1)
14 (12,6)
58 (52.3)
0.025a
a Χ2
c U Mann Whitney
* Defined according to CDC-AAP criterion
Table 2: Periodontal clinical characteristics of the study participants.
Since maternal age, BMI, family history of diabetes and sociocultural level are considered risk factors for GDM. They can act as confounders of the influence of periodontitis (as a global assessment of periodontal health). For this reason, we performed a binary logistic regression and the predictor variables were included together with periodontitis. Only age (OR = 1.14, 95% CI 1.06 to 1.22, p <0.001), educational level (OR = 0.56, 95% CI 0.36 to 0.85, p <0.01) and periodontitis (OR = 2.43, 95% CI 1.32 to 4.45, p <0.01) were significantly associated. In (Figure 1), we show the ROC curve whose values are ROC = 0.73 CI 95% 0.66 to 0.79, p< 0.001.
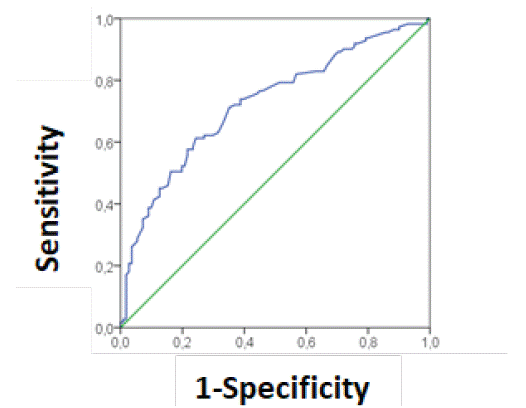
Figure 1: Area under the curve of the diagnostic capacity of GDM based on age, educational level and periodontitis ROC = 0.73 CI 95% 0.66 to 0.79, p< 0.001.
Secondary Results
To study if patients with GDM with or without insulin have different periodontal condition, we compared the periodontal variables. No differences were found between periodontal status and insulin-dependent / non-insulin-dependent diabetes in GDM pregnant (Table 3).
Non-insulin
dependent
n=79Insulindependent n=32
p
Plaque Index
PI = 20 %
PI < 20 %n (%)
40 (50.6)
39 (49.4)
20 (62.5)
12 (57.5)
0.256a
Bleeding on Probing
BOP = 10 %
BOP < 10 %n (%)
58 (73.4)
21 (26.6)26 (81.3)
6 (18.7)0.384a
Pocket Probing Depth
Mean PPD (mm)Mean (SD)
2.34 (0.36)
2.31 (0.28)
0.990c
Clinical Attachment Level
Mean CAL (mm)Mean (SD)
2.39 (0.38)
2.35 (0.29)
0.848c
Periodontal status
Periodontal health
Gingivitis
Periodontitis*
n (%)
15 (19)
11(13.9)
53 (67.1)
3 (9,4)
4 (12.5)
25 (78.1)
0.113a
a Χ2
c U Mann Whitney
* Defined according to CDC-AAP criterion
Table 3: Periodontal status in insulin and non-insulin dependent GDM participants.
No association was found (p < 0.05) between adverse pregnancy outcomes and periodontal condition in diabetes gestational pregnants. (Table 4).
DMG with adverse outcomes
n=46DMG withoutadverse outcomes
n=53p
Plaque Index
PI = 20 %
PI < 20 %n (%)
27 (58.7)
19 (41.3)
28 (52.8)
25 (47.2)
0.558a
Bleeding on Probing
BOP = 10 %
BOP < 10 %n (%)
37 (80.4)
9 (19.6)41 (77.4)
12 (22.6)0.709a
Pocket Probing Depth
Mean PPD (mm)Mean (SD)
2.35 (0.38)
2.31 (0.33)
0.723c
Clinical Attachment Level
Mean CAL (mm)Mean (SD)
2.40 (0.39)
2.38 (0.35)
0.955c
Periodontal status
Periodontal health
Gingivitis
Periodontitis*
n (%)
8 (17.4)
10(21.7)
28 (60.9)
6 (11.3)
5(9.4)
42 (79.3)
0.59a
a Χ2
c U Mann Whitney
* Defined according to CDC-AAP criterion
Table 4: Periodontal condition in GDM participants with and without adverse pregnancy outcomes.
Discussion
Our study shows that pregnant women who developed gestational diabetes during their pregnancy, in addition to being older and with a lower level of education, had a worse periodontal condition than non-diabetic pregnant women. This is because they had fewer teeth, a greater presence of gingival inflammation and periodontitis, as well as a higher average of PPD and CAL. Maternal age, educational level and the presence of periodontitis are independent risk factors for GDM.
The results of this study showed a significant association between having a family history of first degree diabetes and developing GDM. Some studies coincide with these findings [17] while others did find a relationship [18]. As far as BMI is concerned, a BMI ≥ 30 Kg/m² is considered to be a risk factor for developing GDM [19]. Some studies observe the association of BMI with women who had GDM [17,18,20-22]. Our results initially show that a higher BMI occurs among gestational diabetics, however, when performing logistic regression its importance disappears. This could be due to sample limitation. In fact, only 30 patients had a BMI greater than 30, of which 19 had GDM and 11 did not, Χ2 = 1.96, p= 0.16. Our sample size is probably responsible for not including it as a risk factor.
The presence of bacterial plaque is the main risk factor for PD, so it could act as a confounding factor between periodontal parameters and GDM. In this study, it was observed that there were no significant differences in PI between cases and controls, so we ruled out dental plaque as a possible confounder that could exacerbate the relationship between PD and GDM. Bagis et al. [22] also found no differences in PI between the two groups. However, other authors did observe significant differences [21].
Other factors that did not show a significant relationship with GDM and agreeing with what has been observed in the literature are smoking [17,20] and brushing frequency [17,18,23]. When analyzing bleeding on probing between cases and controls, statistically significant differences were found. There were more pregnant women with GDM who presented gingival inflammation than in the control group (75.7% vs 56.8% respectively). These results agree with those found by other authors [17,18,21,22]. Kumar et al. [24] in their work concluded that the incidence of GDM was significantly higher in women with gingivitis, periodontitis and periodontal disease in general compared to women with healthy gums, consistent with our findings.
Regarding the number of teeth, the gestational diabetics showed a significantly lower number than the controls. These results are in line with other studies [20,21]. In this study, the mean periodontal parameters of PPD and CAL are statistically related to GDM. We found significant differences in the mean PPD between the patients of the two groups (p=0.001). We agree with others in the existence of a significantly higher mean PPD in diabetic pregnant women than in controls [17,18,21]. However, Bagis et al. [22] find no relationship between mean PPD and the presence or absence of GDM. Regarding the mean CAL in patients with GDM and without GDM, we found significant differences (p=0.013). Other works such as that of Chokwiriyachit et al. [18] or Ruiz et al. [21] agree with these findings.
The educational level maintained significant differences with the GDM. In this work, it is an independent risk factor for GDM so that, the higher the level of education, the lower the prevalence of GDM. Other works did not take this factor into account [18,21] or found no relationship.[17,20,23]. The level of education can be considered a proxy for the economic situation. A higher level of education is generally associated with a higher economic level, greater health education and access to health care.
Older age is a risk factor for gestational diabetes [17,20,21]. In this study, the women with GDM were significantly older than the pregnant women in the control group. In fact, in our casuistry, age appears as a predictor OR 1.14, p <0.001. The other risk factor that we observed in our study is the presence of periodontitis OR = 2.43, p <0.01, observation confirmed by others [17,18,21]. Interestingly, two systematic reviews, published in the same year, showed contradictory results. Esteves Lima et al. [12] concluded that the scientific evidence could not demonstrate a positive association between periodontitis and GDM. In contrast, the results of the meta-analysis by Abariga and Whitcomb [13] suggested those patients with periodontitis were more than twice as likely to develop GDM, a figure similar to our estimate risk. In the same sense and more recently, Kumar et al [24] observed an association between periodontal disease and GDM and a higher risk of developing preeclampsia, with an adjusted HR of 2.85, 95% CI = 1.47 to 5.53. Esteves Lima et al. [12] observed a significant association in the meta-analysis of 4 cross-sectional studies (OR 1.67, 95% CI 1.20 to 2.32) and also in two case studies (OR 2.66, 95% CI 1.52 to 4.65), while when in this last group they integrate their own study, the significance of risk disappears (OR 1.69, 95% CI 0.68 to 4.21). These same authors point out the difficulty in the study given the clinical, methodological and statistical differences between the studies.
Scientific evidence from numerous studies indicates that diabetes mellitus (DM) and PD are bidirectionally related [25]. In other words, being diabetic is a risk factor for the onset and progression of PD and, although there is less scientific evidence, periodontitis could increase the risk of developing DM and compromise glycemic control in patients with DM. Therefore, in this work we have tried to assess the existence of a possible bidirectional association between GDM and PD. After an extensive review of the literature, we are not aware of studies that analyze whether patients with GDM and worse periodontal condition have worse glycemic control and require more frequent insulin treatment. To study this possible association, we separated the 111 patients with GDM into two groups: insulin dependent (n=32) and non-insulin dependent (n=79) following the ACOG classification. When analyzing the influence of the periodontal parameters: PI, BOP, PPD and NIC and the PD status on the glycemic control of GDM, no differences were observed at the 95% confidence level between insulin-dependent and non-insulin-dependent patients. Therefore, we cannot say that pregnant women with GDM and worse periodontal condition show lower glycemic control than patients with GDM and periodontal health. Other studies should analyze the impact of the periodontal condition on the glycemic control of gestational diabetes.
In this work we have recorded perinatal complications (preterm birth, LBW, PROM, preeclampsia, macrosomia, and IUGR). When reviewing the literature, we verified that only two studies collect the adverse pregnancy outcomes. In the one by Dasanayake et al. [26] where perinatal complications were studied with GDM but not with PD, this author found that pregnant women with GDM presented more PROM. However, this difference was not observed in our study. The other study that analyzed perinatal complications with PD and GDM was that of Kumar et al. [24] who established a relationship between PD and GDM and an increased risk of developing preeclampsia due to this association. In our study we also found that pregnant women with gestational diabetes had a significantly higher risk of preeclampsia. However, our results showed no relationship between preeclampsia and PD. Interestingly, there are studies in which the treatment of periodontitis during pregnancy does not reduce the adverse obstetric effects associated with it, such as prematurity or low birth weight [27,28]. We found no association between the existence of periodontitis and prematurity or preeclampsia. Others do find it, with OR for premature birth and/or low birth weight between 2.04 to 4.19 [29].
Limitations
Among the main limitations of the studies of GDM and periodontitis is the discrepancy of the definitions used to diagnose both GDM and periodontitis. In this work, in addition to analyzing the mean periodontal parameters of PPD and CAL, the CDC-AAP [16] criteria have been used. Although an association between periodontitis and GDM is observed in this study, its cross-sectional design does not allow establishing causality judgments. Whether periodontitisis a risk factor for GDM or whether having GDM or a prediabetic metabolic situation would increase the prevalence and severity of periodontitis.
The number of patients examined, despite being the highest of most relevant publications that aim to relate PD to GDM [17,18,20,24,26] so far, is a limiting factor since it could be insufficient or less likely to find significant differences when analyzing low-prevalence diseases such as preeclampsia or premature birth, which some authors relate to the periodontitis. Another possible limitation is the non-blinded study design.
Conclusions
Patients who develop gestational diabetes have a worse periodontal condition than unaffected ones. The risk of GDM can be estimated in our sample based on periodontitis, age and educational level.
A worse periodontal condition does not imply that pregnant women with GDM require more insulin treatments than gestational diabetic pregnant women with better periodontal status.
Gestational diabetic women with periodontal pathology do not have more perinatal complications than pregnant women with GDM and better periodontal condition.
Acknowledgements
We thank IIS (Instituto de Investigación Sanitaria La Fe, Hospital Universitario y Politécnico La Fe de Valencia) for authorizing and facilitating the clinical study of all the patients. This work has not received external funding. The authors declare no conflicts of interest.
Ethics Approval and Consent to Participate
All patients received the information sheets and signed the consent before their participation in the study. They were also informed of the confidentiality of the data collected following the protocol of the Organic Law on Data Protection (LOPD) 15/1999. The study was carried out following the Declaration of Helsinki, and authorized by the Ethics Committee for Biomedical Research and the Research Commission of the Hospital La Fe in May 2017.
References
- Komine-Aizawa S, Aizawa S, Hayakawa S. Periodontal diseases and adverse pregnancy outcomes. J Obstet Gynaecol Res. 2019; 45: 5-12.
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017; 3: 17038.
- Van Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta- regression. Journal of Dental Research. 2014; 93: 1045-53.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019; 42: S13.
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol. 2007; 47: 307-312.
- Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007; 30: 2287.
- Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019; 42: 27-35.
- Nadeau-Vallee M, Obari D, Palacios J, Brien ME, Duval C, et al. Sterile inflammation and pregnancy complications: A review. Reproduction. 2016; 152: R277–R292.
- Corbella S, Taschieri S, Del Fabbro M, Francetti L, Weinstein R, Ferrazzi E. Adverse pregnancy outcomes and periodontitis: A systematic review and meta-analysis exploring potential association. Quintessence Int. 2016; 47: 193-204.
- Daalderop LA, Wieland BV, Tomsin K, Reyes L, Kramer BW, Vanterpool SF, Been JV. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin Trans Res. 2018; 3: 10-27.
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal Medicine: 100 Years of Progress. J Dent Res. 2019; 98: 1053-1062.
- Esteves Lima RP, Cyrino RM, de Carvalho DB, Oliveira da Silveira J, Martins CC, Miranda Cota LO, Costa FO. Association between periodontitis and gestational diabetes mellitus: systematic review and meta-analysis. J Periodontol. 2016; 87: 48–57.
- Abariga SA, Whitcomb BW. Periodontitis and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Observational Studies. BMC Pregnancy and Childbirth. 2016; 16: 344.
- Harper LM, Mele L, Landon MB, Carpenter MW, Ramin SM, et al. Carpenter-Coustan Compared With National Diabetes Data Group Criteria for Diagnosing Gestational Diabetes. Obstet Gynecol. 2016; 127: 893.
- Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: Case definition and diagnostic considerations. J Periodontol. 2018; 89: S46-S73.
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2012; 83: 1449–1454.
- Xiong X, Elkind-Hirsch KE, Vastardis S, Delarosa RL, Pridjian G, Buekens P. Periodontal disease is associated with gestational diabetes mellitus: a case–control study. J Periodontol. 2009; 80: 1742–9.
- Chokwiriyachit A, Dasanayake AP, Suwannarong W, Hormdee D, Sumanonta G, et al. Periodontitis and gestational diabetes mellitus in non-smoking females. J Periodontol. 2013; 84: 857-862.
- Committee on Practice Bulletins Obstetrics. ACOG PracticeBulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018; 131: e49-e64.
- Esteves Lima RP, Miranda Cota LO, Costa FO. Association between periodontitis and gestational diabetes mellitus: a case–control study. J Periodontol. 2013; 84: 1257–65.
- Ruiz DR, Romito GA, Dib SA. Periodontal disease in gestational and type 1 diabetes mellitus pregnant women. Oral Dis. 2011; 17: 515-521
- Bagis N, Bostanci HS. The relationship between gestational diabetes mellitus and periodontal health: a case–control study. Int J Exp Dent Sci. 2013; 2: 71.
- Poulsen H, Meurman JH, Kautiainen H, Heikkinen AM, Huvinen E, et al. Oral Health in Women with a History of High Gestational Diabetes Risk. Dent J (Basel). 2019; 7: 92.
- Kumar A, Sharma DS, Verma M, Lamba AK, Gupta MM, et al. Association between periodontal disease and gestational diabetes mellitus: a prospective cohort study. J Clin Periodontol. 2018; 45: 920-931.
- Montero E, Madianos P, Herrera D. Diabetes y enfermedades periodontales: su asociación bidireccional y sus implicaciones. Periodoncia Clínica. 2017; 8, 35-49.
- Dasanayake AP, Chhun N, Tanner ACR, Craig RG, Lee MJ, et al. Periodontal pathogens and gestational diabetes mellitus. J Dent Res. 2008; 87: 328–33.
- Caneiro-Queija L, López-Carral J, Martin-Lancharro P, Limeres-Posse J, Diz-Dios P, Blanco-Carrion J. Non-Surgical Treatment of Periodontal Disease in a Pregnant Caucasian Women Population: Adverse Pregnancy Outcomes of a Randomized Clinical Trial. Int J Environ Res Public Health. 2019; 16: 3638.
- da Silva HEC, Stefani CM, de Santos Melo N, de Lima AA, Rosing CK, et al. Effect of intra-pregnancy nonsurgical periodontal therapy on inflammatory biomarkers and adverse pregnancy outcomes: a systematic review with meta-analysis. Syst Rev. 2017; 6: 197.
- Teshome A, Yitayeh A. Relationship between periodontal disease and preterm low birth weight: systematic review. Pan Afr Med J. 2016; 24: 215.