Research Article
Austin J Dent. 2014;1(1): 1004.
Effects of Carbonated Cola Beverages, Sports and Energy Drinks and Orange Juice on Primary and Permanent Enamel Dissolution
Owens BM1*, Mallette JD2 and Phebus JG3
1Department of Restorative Dentistry, University of Tennessee Health Sciences Center, USA
2Private Practices, Pediatric Dentistry, Murfreesboro, USA
3Department of Endodontics, University of Tennessee Health Sciences Center, College of Dentistry
*Corresponding author: Owens BM, Department of Restorative Dentistry, University of Tennessee Health Sciences Center, College of Dentistry, 875 Union Avenue, Memphis, TN 38163, USA
Received: May 28, 2014; Accepted: June 14, 2014; Published: June 17, 2014
Abstract
The purpose of this in vitro study was to evaluate the effect of carbonated soft drinks, sports and energy beverages, orange juice, and tap water on primary and permanent enamel specimens, measuring mean percentage weight loss.
Beverages used in this study included: Coca-Cola Classic, Diet Coke, Gatorade sports drink, Minute Maid Pure Premium Orange Juice, Red Bull energy drink, and tap water (control). Extracted primary and permanent teeth sectioned into uniform slabs. Twelve primary and 30 permanent enamel specimens were randomly distributed to six beverage groups. The specimens were immersed in each beverage for 24-hour intervals for a 10-day period at 37°C. Specimens were thereafter weighed following each immersion period, with mean percent weight losses calculated per beverage group. The pH and titratable acidity was also determined for each beverage. Enamel weight loss data was subjected to statistical analysis at p<0.05 level of significance.
Primary and permanent enamel specimens immersed in Red Bull and Gatorade showed the greatest mean percent weight loss. Percent weight loss of both primary and permanent specimens showed linear progression with time.
The findings indicate that energy and sports drinks, displayed significantly greater percent mean weight losses of the primary and permanent enamel (dentin) specimens.
These results suggest that intake of these beverages cause enamel dissolution with an accompanying clinical diagnosis of dental erosion. Caution should be exercised in the excessive consumption of these beverages, especially by children and adolescents.
Keywords: Erosion; Soft drinks; Sports drinks; Energy beverages; Ph, Titratable acidity
Introduction
In the last three decades, the consumption by children and adolescents of "Sugary" or "Sugar-sweetened Beverages" (SSBs) including carbonated "soda" soft drink, sports and energy beverages, fruit drinks, and sweetened bottled waters have increased dramatically [1-7]. Although consumption of carbonated soft drinks has been declining since 2005, U.S. consumers spent a total of $29.0 billion dollars on all SSBs in 2011, with an average consumption of 45 gallons per person per year [8,9].
Since their introduction, consumption of "diet" soft drinks, containing caffeine, artificial sweeteners, and other acidic ingredients have accounted for 29 percent of the soft drink market; however, sales of these drinks have also declined [2]. "Diet" soft drinks were first marketed in the 1980's as low (no) calorie alternatives to "classic" or traditional carbonated cola formulations as "diet" alternatives containing many different flavor choices [10].
So what segment of the population consumes the majority of these beverages? Yes, children and adolescents [11]. At least one SSB is consumed by 66% of children and 77% of adolescent's daily [8]. Including adults and adolescents, males have been found to consume more SSBs than females [3]. Older females consume the lowest (42 calories/day) amount, with the highest consumption rates (70%) by male adolescents, 12-19 years old (273 calories/day) [3]. Furthermore, dietary surveys of adolescents in 1994 indicated that only 29% of boys and 12% of girls consumed the recommended intake of dairy foods. As a result, only 36% of boys and 14% of girls consumed 100% of the Recommended Dietary Allowance (RDA) of calcium, a necessary nutrient for the maintenance of teeth (enamel) and bones [12,13]. In comparison, according to data from 1977- 78, boys and girls consumed 50% more milk than soft drinks as compared to 1994-96 when the beverage consumption levels were reversed [14]. Between 1965 and 1994-96, calcium consumption in children age 11 to 18 decreased from 1,100 mg to 960 mg per day [15]. Thus an increased intake of soft drinks has translated into children and adolescents receiving too little calcium; vitamins A, B and C and magnesium in their diets [16,17]. Consumption of SSBs has also been associated with poor dietary habits, weight gain, obesity, and type 2 diabetes, predominately in adults, but also recognized in children and adolescents [3,5,18-21].
As previously stated, consumption of carbonated soft drinks (sweetened cola's and diet cola's) has decreased, in favor of sports and energy beverages and sweetened waters [5,10]. Sports drinks were originally created as carbohydrate and electrolyte aqueous formulations, to supplement performance and to prevent dehydration during strenuous exercise among.
Athletes [5,7]. From their inception in the 1960's, sports drink consumption has increased significantly, with sales in the U.S. topping $1.5 billion dollars yearly (2008 sales of $7.5 billion); with one report concluding that between 51-62% of adolescents drink at least one sports drink per day [5,22-24]. Consumption trends note that between 2002-2004, among school-age adolescents, the purchase of carbonated soft drinks fell 24% while sports drinks consumption rose by 70% [25]. According to one report [26], "55% of middle school students and 80% of high school students purchased sports drinks (at school).
A second segment of non-traditional beverages marketed to, primarily, the 18-35 age groups include energy or "high-energy" drinks [27]. These beverages, contain stimulants, carbohydrates, amino acids, proteins, vitamins and minerals, and additional acids and other ingredients, with claims of enhancement of physical and mental performance [6,7]. These beverages appeal to younger age groups through anachronistic advertising labels and "hip", new generation slogans, as well as to adults by touting claims of increased short-term energy bursts and increased concentration while at the workplace. According to Beverage World, consumption of energy drinks in the U.S. went from "59.5 million gallons per year in 2003 to 354.5 million gallons in 2009" [28]. Reports in the literature have shown that over 40% of adolescents consume energy drinks and that $3 billion was spent on these beverages in 2008 by adolescents aged 12-24 [29,30]. A correlation between excessive consumption of all the fore-mentioned beverages and the loss of, particularly enamel, following intake, is well established in the dental literature [31,32]. Of particular relevance is the loss of tooth structure through a chemical process of dissolution, whereby extrinsic substances cause irreversible loss of dental hard tissue (enamel and dentin) without the involvement of microorganisms, with a clinical diagnosis of erosion [33,34].
In the oral environment many factors can modify the effect of SSB ingredients on tooth structure, including the chemical properties of the beverage ingredients (pH, titratable acidity, and electrolyte [calcium and phosphate] concentrations); the frequency and method of contact between the enamel surface and the solution; salivary composition, buffering capacity, and flow rate; pellicle formation; enamel type; and individual drinking habits and oral hygiene [35].
The authors recognized that in vitro testing protocols conducted in the present study cannot fully replicate and possibly over-predict in vivo beverage consumption practices and the many factors associated with salivary function. However, the experimental protocol performed (percent weight loss of primary and permanent enamel by select beverages) could be predictive of formal "erosive" processes found in the oral cavity, prior to the execution of further indebt and perhaps more realistic longitudinal, in vivo studies. Consequently, the purpose of this study was to measure the percent weight loss of primary and permanent enamel (and dentin) specimens following immersion in different beverages.
Materials and Methods
Six test beverages (SSBs, fruit juice, and water) were chosen for inclusion into the present study (Table 1). Individual beverages tested were from the same batch as to insure consistency for the testing protocol. Recently extracted maxillary and mandibular primary and permanent human teeth, free of hypo calcification, caries, and macroscopic fractures were carefully cleaned of calculus and other debris. The teeth were previously stored in a 1% Chloramine-T solution (Fisher Chemical, Fair lawn, NJ, USA) consisting of 12% active chlorine diluted in distilled water. The facial or lingual surfaces of all teeth were sectioned into uniform segments (approximately 4.0 mm x 6.0 mm x 1.5 mm) utilizing a high-speed, water-cooled hand piece with a straight fissure carbide bur, and stored in tap water immediately prior to experimentation. It must be noted that it was impossible to isolate only enamel from dentin hard tissues in all of the primary specimens; therefore, all primary teeth contained small remnants of dentin tissue. Primary tooth specimens were divided among five beverage groups and tap water (control) and placed into separate opaque containers, with one specimen per container. Due to the limited availability of sound primary teeth for inclusion in this study, only 12 total specimens could be obtained and sectioned. Permanent enamel test specimens were also randomly distributed to the same beverage groups (and tap water), comprising 5 specimens per group and placed in separate containers, again, with one specimen per container. Prior to specimen immersion, each beverage was tested for: 1) pH (in triplicate) utilizing a pH electrode connected to a Jenco 3601™ (Tenco Instruments Inc., San Diego, CA) analyzer, and 2) titratable acidity (TA) (in triplicate) - titrated to a pH of 8.3 utilizing 1.0 N NaOH. The TA was performed on each beverage by titration of the weight in grams (g) of 1.0 N NaOH required to raise the pH to a level of 8.3.
Group
Beverage Manufacturer
Composition
pH (s.d.)
TA (s.d.)
1
Coca-Cola Classic (Coke)
The Coca-Cola Co. Atlanta, GA
Carbonated water, High fructose corn syrup, Caramel color, Phosphoric acid, Natural flavors, Caffeine
2.49 (.006)
9.57 (1.87)
2
Diet Coke
The Coca-Cola Co. Atlanta, GA
Carbonated water, Caramel color, Aspartame, Phosphoric acid, Potassium benzoate, Natural flavors, Citric acid, Caffeine
3.16 (.015)
9.11 (1.63)
3
Gatorade
The Gatorade Co. Water, Chicago, IL
Sucrose syrup, Glucose-fructose syrup, Citric acid Natural lemon/lime Flavors, Natural flavors, Salt, Sodium Citrate, Monopotassium phosphate, Ester gum, Yellow
3.04 (.006)
10.26 (1.18)
4
Minute Maid
Atlanta, GAThe Coca-Cola Co.
Pure filtered water, premium concentrated orange juice Pure Premium Orange Juice (OJ)
3.86 (.006)
26.03 (3.46)
5
Red Bull
Red Bull N.A., Inc. Santa Monica, CA
Water, Sucrose, Glucose, Sodium citrate, Taurine Glucuronolactone, Caffeine, Inositol, Niacinamide, Calcium-Pantothenate, Pyridoxine HCL, Vitamin B12,
3.32 (.006)
28.99 (4.17)
6
Tap Water
(Water)N/A
Water, various minerals
7.55 (.010)
----------
Table 1: Beverage Characteristics.
A portion of the testing protocol was adopted from a study as reported by von Fraunhofer & Rogers [36]. It must also be noted that representative primary and permanent specimens were placed in a desiccation chamber for 24-hours, weighed using a calibrated digital balance, followed by placement in a container of tap water for 24-hours and, again, weighed. This procedure was performed in order to test the possible effects of water absorption by the enamel specimens. It was found that the specimen weights were not significantly different following both desiccation and water immersion procedures.
Initial weights of all primary and permanent tooth specimens were performed prior to beverage immersion. Containers were filled with the respective beverage, labeled, and sealed, with the temperature maintained at 37°C. After 24 hours, each specimen was removed from the beverage using tweezers, rinsed with tap water, blotted dry with paper towels, left undisturbed to dry for 15 minutes, and weighed. The weight values were recorded daily. The weighing process was repeated every day for 10 days and beverages were changed daily. Since all of the specimens originally varied slightly in size and weight, the percentage of weight loss was calculated. Weight loss data were subjected to Analysis Of Variance (ANOVA) and post hoc Fisher's testing at p<0.05 level of significance. The statistical analyses were completed using Statview 5.0 (SAS Institute, Cary, NC).
Results
The pH and TA measurements for each beverage are also shown in (Table 1) according to ANOVA analysis, significant (p<.0001) differences were exhibited between the tested beverages regarding pH, with ad hoc Fisher's testing indicating significant differences between all paired groupings (beverages). Coke had the lowest mean pH value of 2.49, while tap water (Water) revealed the highest mean pH of 7.55. Titration results (compacted or combined readings from each pH level) also showed significant (p<.0001) differences between the beverages. Specimens immersed in Red Bull showed the greatest TA with a mean of 28.99 g, while Diet Coke had the lowest mean TA value of 9.11 g.
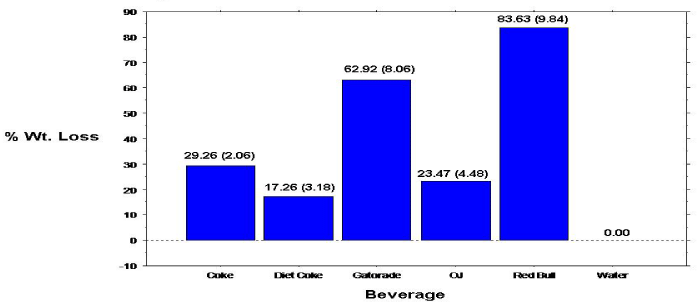
Figure 1: Percent Weight loss of primary teeth enamel specimens.
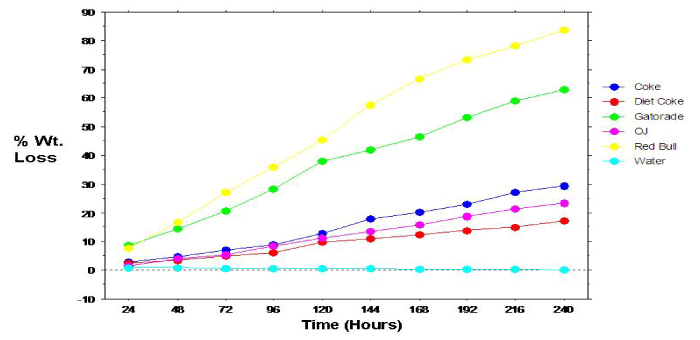
The overall mean 10-day mean percent weight loss calculations for the primary tooth specimens are shown in Figure 1. Analyses revealed statistically significant (p=.0005) differences comparing the overall mean percent weight loss for the individual beverage/ specimens. Specimens immersed in Red Bull exhibited significantly greater mean percent weight loss compared to all other beverage/ specimens, followed by specimens immersed in Gatorade. Paired groupings, except Coke & Diet Coke; Coke & OJ; Diet Coke & OJ; and Gatorade & Red Bull, showed significant differences in mean percent specimen weight loss. Percent weight loss of the permanent specimens also progressed linearly with time (Figure 2).
Figure 2: Relation between dissolution of primary teeth specimens and time.
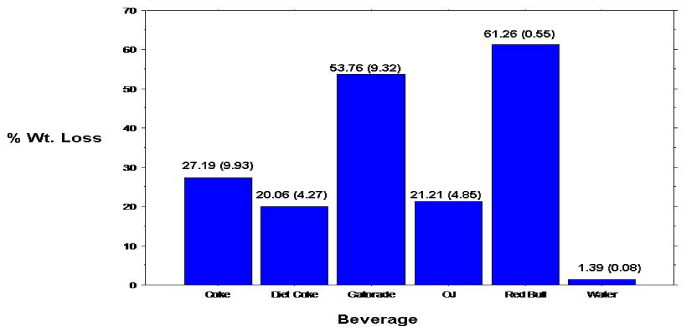
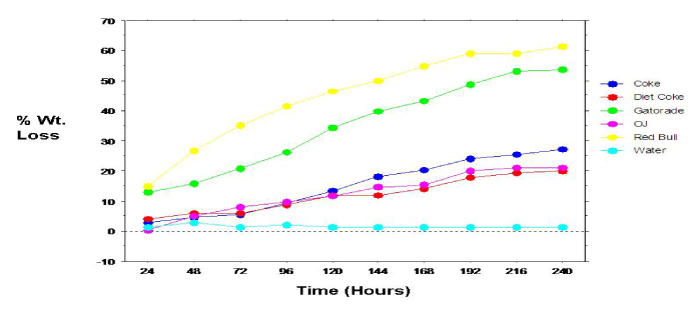
The overall mean 10-day mean percent weight loss was calculated for the permanent (tooth) specimens (Figure 3). Analyses revealed many of the same results (compared to the primary specimens), with statistically significant (p<0.0001) differences, between the mean percent weight loss for the individual beverage/specimens. Specimens immersed in Red Bull revealed the greatest mean percent weight loss with a reading of 83.63%. Specimens immersed in Diet Coke showed the lowest mean percent weight loss (excluding tap water with a 0% mean weight loss). All paired beverage groupings, except Coke & OJ and Diet Coke & OJ revealed significant differences in mean percent weight loss. Again, as evidenced with the primary (tooth) specimens, mean percent weight loss among permanent (tooth) specimens progressed linearly with time (Figure 4). Because the specimen samples (count) among the primary and permanent teeth were not equal, a statistical comparison between samples were not conducted.
Figure 3: Percent weight loss of permanent teeth enamel specimens.
Figure 4: Relation between dissolution of permanent teeth specimens and time.
Discussion
Although this study was conducted in vitro, the results suggest that popular SSBs as well as fruit juices have the potential to alter tooth structure. This study demonstrated the mean percent tooth weight loss of the beverage affected specimens, but did not provide any direct evidence to demonstrate that a formal diagnosis of clinical "erosion" occurred. However, the study did reveal that enamel (dentin) dissolution did occur as the primary and permanent specimens weighed less following the 10-day immersion period in each respective beverage.
As previously stated, several factors play important roles in the potential destruction of tooth structure following exposure to soft drinks and other sweetened beverages [35]. The pH value is a measure of the initial hydrogen ion concentration, while the titratable acidity or buffering capacity is the total number of acid molecules, and determines the actual hydrogen ion availability for interaction with the tooth surface [37]. Salivary pH lies within a range of 5.5 to 6.5, with a pH of 5.5 or below, accepted as a threshold level for destruction of tooth structure, i.e. caries and erosion [38]. A sustained low salivary pH (<5.5) has been shown to be a result of intake of carbohydrates or sugars (sucrose, fructose), and acids (phosphoric, citric, and other organic acids) [37]. These ingredients decrease the buffering capacity (of saliva) and maintain the oral pH below the threshold level (5.5 pH) necessary for alteration of enamel [39]. Acid accumulation at the tooth surface can result in an immediate drop in pH, but tooth dissolution is minimized due to the actions of salivary proteins and the acquired pellicle which dilute the remaining acids following deglutition. This process of diluting and washing away the potentially harmful effects of acids occurs until the oral pH rises above the critical 5.5 pH threshold. A constant assault by acids or additives from continued exposure (as conducted in this study), consumption by athletes, or incorrect drinking habits can lead to a breakdown in this process resulting in accelerated demineralization of the enamel (and dentin) surface [40]. Fortification of SSBs and even fruit juices with calcium, phosphorous, and fluoride have been shown to be limiting factors of the erosion potential in the oral cavity [41,42]. In the present study, Coke caused increased percent weight loss of the enamel specimens compared to Diet Coke. This finding suggests that beverages such as Coke, supplemented with refined carbohydrates or sugars (sucrose, high fructose corn syrup), compared to artificial sweeteners found in diet versions of the same beverage, may be contributing factors to tooth dissolution. It must be noted that during the titration process, the more titration of a neutral agent required, the longer it will take for salivary components to neutralize the acid, with a corresponding increase of the erosion potential of tooth structure [37].
The results of this study also revealed that primary and permanent enamel specimens immersed in Red Bull exhibited the greatest percent weight loss. Although the pH of Red Bull was higher than many of the other beverages, the titration results revealed that Red Bull exhibited a significantly greater TA compared to the other beverages. Interestingly, the readings attained by the Gatorade showed a relatively low pH (3.03) and TA (10.27) values; however, enamel specimens immersed in this drink displayed a significantly greater percent weight loss, second only to Red Bull. A possible explanation could be the effect of included additives, such as refined sugar syrups and sodium citrate, contained in Gatorade and the destructive reaction of these ingredients or combination of ingredients to enamel substrate. The significantly greater specimen percent weight loss shown by the sports and energy drinks compared to the two cola beverages suggests that the increased potential for enamel weight loss or dissolution resulted from the effects other than actual pH, possibly through the binding or chelation and eventual loss of calcium ions.
Phosphoric, citric acid and/or citrates found in many soft drinks are added as flavoring agents or acidulants, and can concurrently chelate (bind) to calcium, removing the beneficial effects of calcium in the mineralization process, promoting an decreased buffering effect of saliva and thus increased tooth destruction [35,37]. As these acids lower the pH of the saliva, calcium ions are extracted from the tooth structure (enamel/dentin) into the saliva to compensate for this low oral pH environment. This process leaves a softened matrix for additional destruction by the caries process or by mechanical abrasion. It has been shown that calcium and phosphate supplements are often added by manufacturers to counteract for the initial loss of calcium [42]. These previously reported conclusions somewhat corroborated the results of the present study, whereby specimens immersed in beverages (Red Bull and Gatorade) containing citric acid (citrate) and/or fruit-based sugar ingredients showed greater tooth percent weight loss. Red Bull and Gatorade contain sodium citrate (sodium salt of citric acid), a buffering agent thought to aid in maintaining the pH levels in soft drinks; however, sodium citrate is a sequestering agent that binds to calcium.
As shown by the progressive weight loss of the primary and permanent enamel specimens over a 10-day period, contributing factors could include: a persistent leaching of calcium ions from the tooth structure, concurrently affecting the buffering capacity, allowing the "low pH" beverages to cause further destruction and, in turn, greater percent weight loss. According to one study [43], with the higher concentration of calcium ions found in soft drinks, the less likely the enamel surface calcium ions will be detached. Another study, [44] using atomic absorption as a measuring device, found a direct relationship between the weight loss of tooth enamel and loss of calcium ions. Still yet another study [41] has indicated that the erosive potential occurs in the first few minutes following exposure and is a factor of the beverage pH.
Other investigations [36,45-48] evaluating the "erosive potential" of various beverages on permanent tooth structure revealed similar results compared to the present study. Separate studies [32,49] evaluating the surface roughness of enamel specimens following immersion in various beverages indicated that Red Bull, Gatorade, and Coke showed the greatest degree of enamel surface roughness.
Soft drinks, sports and energy beverage ingredients can be especially destructive to children's and adolescent's teeth since mineralization in immature permanent enamel is not complete, allowing an increased susceptibility from the aggressive nature (of these beverages) [50]. However, previous research [51,52] has shown inconclusive results, comparing primary and permanent enamel dissolution levels. In the present study, the percent weight loss of the primary enamel specimens was lower (although not shown statistically) compared to the permanent enamel counterparts. Primary tooth enamel has a higher degree of porosity and a lower degree of mineralization than permanent enamel, suggesting that primary enamel is more susceptible to the effects of soft drinks [48]. A study by Johansson et al. [53] investigating the susceptibility of primary and permanent enamel to citric acid showed primary enamel more susceptible to dissolution than the permanent counterpart. In this study, primary enamel specimens included small portions of dentin tissue which could have affected the percent weight loss. A possible reason for the decreased percent weight loss of the primary enamel could have been a result of the buffering properties of the organic components of dentin, with the collagen content serving as a diffusion barrier to the low pH environment of soft drinks [54,55].
In the present experimental model the amount of beverage was constant in relation to absence of a modifying agent (saliva), and thus the rate of dissolution was evident, and often extreme, as revealed with specimen immersion in Red Bull and Gatorade. Each beverage had constant contact with the specimens over a 10-day testing period and did over-estimate the amount of tissue loss compared to conditions in the oral environment.
It is very difficult to replicate in vivo conditions of the oral environment or to directly correlate the present results to the effects of beverage consumption in humans. The results of the present study do suggest the potential harmful effects of the tested beverage could be especially pertinent to persons with systemic conditions (Sjögrens syndrome) or to athletes, whereby salivary flow is reduced or non-existent causing xerostomia or dry-mouth conditions [56]. According to one study, "saliva is the most important biological factor affecting the progression of dental erosion" [57]. Human saliva contains hundreds of proteins that serve as protective, modifying factors. Saliva also serves as a buffering agent, diluent, and repository of calcium and phosphate for remineralization - limiting the erosive potential associated with soft drink consumption [57,58]. Attributes of salivary factors in association with the clearance of these beverages is certainly an area of further in vivo research.
Consumers as well as dental professionals should be aware that SSBs and even fruit juices contain ingredients that are potentially harmful, to tooth enamel. This information is of particular importance for children and adolescents with primary and immature permanent enamel; athletes, and persons with systemic problems causing xerostomia, whereby consumption of these beverages should be consumed in moderation. As previously revealed, excessive consumption of SSBs with a concurrent reduction in the dietary intake of dairy products (milk) can only further affect the amount of calcium ions available, necessary for strong enamel.
Conclusion
Within the limitations of the present study, Red Bull and Gatorade showed significantly higher levels of enamel mean percent weight loss compared to the other beverages, although the pH values for these drinks were significantly higher than Coke, Diet Coke, and OJ. These results link beverage ingredients, possibly refined carbohydrates and/or acids, to a sustained low (enough) pH (below the 5.5 pH threshold) for extended periods of time, although the effects of saliva on beverage dissolution of tooth structure was not explored). As a result, the present study attempted to correlate enamel percent weight loss or dissolution to a clinical diagnosis of enamel erosion.
Clinical Significance
Constant exposure to SSBs and fruit juices can potentially cause irreversible loss of tooth structure, especially in children and adolescents. Conditions where salivary production is impaired, as in patients with systemic disease (xerostomia) or in athletes (dehydration), can also place these individuals at possible greater risk for enamel damage or loss.
References
- Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988-2004. Pediatrics. 2008; 121: e1604-1614.
- Economic Research Service, U.S. Department of Agriculture. Beverages, per capita consumption, 1970-2000.
- Ogden CL, Kit BK, Carroll MD, Park S. Consumption of sugar drinks in the United States, 2005-2008. NCHS Data Brief. 2011; 1-8.
- Saad L. Nearly half of Americans drink soda daily. Gallup's annual Consumption Habits Poll. 2014.
- Robert Wood Johnson Foundation, Healthy Eating Research "Consumption of sports drinks by children and adolescents". 2012; 1-7.
- Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007; 6: 35.
- Committee on Nutrition and the Council on Sports Medicine and Fitness. Sports drinks and energy drinks for children and adolescents: are they appropriate? Pediatrics. 2011; 127: 1182-1189.
- Andreyeva T, Chaloupka FJ, Brownell KD. Estimating the potential of taxes on sugar-sweetened beverages to reduce consumption and generate revenue. Prev Med. 2011; 52: 413-416.
- Harris JL, et al. Sugary drink facts: Evaluating sugary drink nutrition and marketing to youth. Rudd Center for Food Policy and Obesity. 2011.
- Beverage Digest. 2013; 1-2.
- US Federal Trade Commission. Marketing food to children and adolescents: A review of industry expenditures, activities, and self-regulation. Washington, DC: US Federal Trade Commission. 2008.
- Food Surveys Research Group, Agricultural Research Service, U.S. Department of Agriculture. Pyramid Servings Data: Results from USDA's 1995 and 1996 continuing Survey of Food Intakes by individuals. 1996 Pyramid servings, tables 2B, 3B, 4B. 1997.
- Food Surveys Research Group, Agricultural Research Service, U.S. Department of Agriculture. Results from USDA's 1994-96 Continuing Survey of Food Intakes by Individual and 1994-96 Diet and health Knowledge Survey. Table 3. 1997.
- Jacobson MF. Liquid candy: How soft drinks are harming America's health. Center for Science in the Public Interest, 2nd ed. Washington, DC. 2005.
- Cavadini C, Siega-Riz AM, Popkin BM. US adolescent food intake trends from 1965 to 1996. Arch Dis Child. 2000; 83: 18-24.
- Ballew C, Kuester S, Gillespie C. Beverage choices affect adequacy of children's nutrient intakes. Arch Pediatr Adolesc Med. 2000; 154: 1148-1152.
- Bowman S. Diets of individuals based on energy intakes from added sugars. Fam Econ Nutr Rev. 1999; 12: 31.
- Babey SH, Jones M, Yu H, Goldstein H. Bubbling over: soda consumption and its link to obesity in California. Policy Brief UCLA Cent Health Policy Res. 2009; 1-8.
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012; 346: 7492.
- Nissinen K, Mikkilä V, Männistö S, Lahti-Koski M, Räsänen L, Viikari J, et al. Sweets and sugar-sweetened soft drink intake in childhood in relation to adult BMI and overweight. The Cardiovascular Risk in Young Finns Study. Public Health Nutr. 2009; 12: 2018-2026.
- Viner RM, Cole TJ. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int J Obes (Lond). 2006; 30: 1368-1374.
- Burke LM, Read RS. Dietary supplements in sport. Sports Med. 1993; 15: 43-65.
- Coombes JS. Sports drinks and dental erosion. Am J Dent. 2005; 18: 101-104.
- Zmuda N. What's a sport? Gatorade redefines to broaden target. Advert Age. 2010; 81: 3-22.
- Wescott RF. Measuring the purchases of soft drinks in US schools: an analysis for the American Beverage Association. 2005.
- Bridging the gap. Trends in student access to competitive venue beverages: Findings from US secondary school.
- Blue-collar soda? Yeah, we've got that. Consumer Reports 2006: 60.
- Beverage World. State of the industry 2007, Chicago; Beverage World. State of the industry 2008, Chicago; Beverage World. State of the industry 2009, Chicago; Beverage World. State of the industry 2010. Liquid refreshment beverages. Chicago.
- O'Dea JA. Consumption of nutritional supplements among adolescents: usage and perceived benefits. Health Educ Res. 2003; 18: 98-107.
- Parker-Pope T. Taste for quick boost tied to taste for risk. The New York Times. 2008.
- Wang LJ, Tang R, Bonstein T, Bush P, Nancollas GH. Enamel demineralization in primary and permanent teeth. J Dent Res. 2006; 85: 359-363.
- Kitchens M, Owens BM. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J Clin Pediatr Dent. 2007; 31: 153-159.
- Ganss C. Definition of erosion and links to tooth wear. Monogr Oral Sci. 2006; 20: 9-16.
- Shipley S, Taylor K, Mitchell W. Identifying causes of dental erosion. Gen Dent. 2005; 53: 73-75.
- Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: a review of the current literature. J Dent. 2006; 34: 2-11.
- Von Fraunhofer JA, Rogers MM. Dissolution of dental enamel in soft drinks. Gen Dent. 2004; 52: 308-312.
- Edwards M, Creanor SL, Foye RH, Gilmour WH. Buffering capacities of soft drinks: the potential influence on dental erosion. J Oral Rehabil. 1999; 26: 923-927.
- Anderson P, Hector MP, Rampersad MA. Critical pH in resting and stimulated whole saliva in groups of children and adults. Int J Paediatr Dent. 2001; 11: 266-273.
- Davani R, Walker J, Qian F, Wefel JS. Measurement of viscosity, pH, and titratable acidity of sports drinks. J Dent Res. 2003; 82.
- Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006; 85: 226-230.
- Davis RE, Marshall TA, Qian F, Warren JJ, Wefel JS. In vitro protection against dental erosion afforded by commercially available, calcium-fortified 100 percent juices. J Am Dent Assoc. 2007; 138: 1593-1598.
- Barbour ME, Shellis RP, Parker DM, Allen GC, Addy M. Inhibition of hydroxyapatite dissolution by whole casein: the effects of pH, protein concentration, calcium, and ionic strength. Eur J Oral Sci. 2008; 116: 473-478.
- Hemingway CA, Parker DM, Addy M, Barbour ME. Erosion of enamel by non-carbonated soft drinks with and without toothbrushing abrasion. Br Dent J. 2006; 201: 447-450.
- Low IM, Alhuthali A. In-situ monitoring of dental erosion in tooth enamel when exposed to soft drinks. Mater Sci Engineer. 2008; 28: 1322-1325.
- Grobler SR, Senekal PJ, Laubscher JA. In vitro demineralization of enamel by orange juice, apple juice, Pepsi Cola and Diet Pepsi Cola. Clin Prev Dent. 1990; 12: 5-9.
- Jain P, Nihill P, Sobkowski J, Agustin MZ. Commercial soft drinks: pH and in vitro dissolution of enamel. Gen Dent. 2007; 55: 150-154.
- Von Fraunhofer J, Barnes A, Barnes D. Enamel dissolution in citric acid-containing beverages. J Dent Res. 2006.
- Ehlen LA, Marshall TA, Qian F, Wefel JS, Warren JJ. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008; 28: 299-303.
- Owens BM, Kitchens M. The erosive potential of soft drinks on enamel surface substrate: an in vitro scanning electron microscopy investigation. J Contemp Dent Pract. 2007; 8: 11-20.
- Lopes GC, Thys DG, Klaus P, Oliveira GM, Widmer N. Enamel acid etching: a review. Compend Contin Educ Dent. 2007; 28: 18-24.
- Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Erosion of deciduous and permanent dental hard tissues in the oral environment. J Dent. 2000; 28: 257-263.
- Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Relative susceptibility of deciduous and permanent dental hard tissues to erosion by a low pH fruit drink in vitro. J Dent. 2000; 28: 265-270.
- Johansson AK, Sorvari R, Meurman JH. In vitro effect of citric acid on deciduous and permanent enamel. Caries Res. 1998; 32: 310.
- Ogaard B, Rølla G, Arends J. In vivo progress of enamel and root surface lesions under plaque as a function of time. Caries Res. 1988; 22: 302-305.
- Ganss C, Klimek J, Brune V, Schürmann A. Effects of two fluoridation measures on erosion progression in human enamel and dentine in situ. Caries Res. 2004; 38: 561-566.
- Stewart CM, Berg KM, Cha S, Reeves WH. Salivary dysfunction and quality of life in Sjögren syndrome: a critical oral-systemic connection. J Am Dent Assoc. 2008; 139: 291-299.
- Hara AT, González-Cabezas C, Creeth J, Zero DT. The effect of human saliva substitutes in an erosion-abrasion cycling model. Eur J Oral Sci. 2008; 116: 552-556.
- Buzalaf MA, Hannas AR, Kato MT. Saliva and dental erosion. J Appl Oral Sci. 2012; 20: 493-502.