
Review Article
Austin J Dent. 2023; 10(1): 1170.
Factors of Obesity Affecting Periodontitis
Yan Y1, Wang W1, Yu F1 and Su S1,2*
1School of Stomatology, Lanzhou University, Lanzhou 730000, China
2Healthy Examination & Management Center, First Hospital of Lanzhou University, Lanzhou 730000, China
*Corresponding author: Su SSchool of Stomatology, Lanzhou University & Healthy Examination & Management Center, First Hospital of Lanzhou University, Lanzhou 730000, China
Received: January 31, 2023; Accepted: March 08, 2023; Published: March 15, 2023
Abstract
Obesity is a systemic chronic low-grade inflammatory reaction, a chronic oxidation process that can damage the body’s physiological functions and lead to various diseases. Periodontitis is the second largest disease affecting oral health and is also harmful to general health. Research has found that obesity provides an inflammatory environment for periodontitis and plays an important role in the occurrence and development of periodontitis. This article mainly reviewed several factors that obesity affects periodontitis, including adipose factors, inflammatory reaction, immune reaction, oxidative stress, insulin resistance, and the increase of periodontal pathogens caused by obesity. Therefore, in treating obesity, we also need to pay attention to periodontal health.
Keywords: Obesity; Periodontitis; Factors
Obesity
Obesity is a chronic disease which has become a public security issue. When the body takes in more calories than it consumes, the excess calories are stored in the body as fat, and it becomes obese when it reaches a certain value. Obesity is related to many factors, such as genetic, neuropsychiatric, endocrine, environmental, and so on. Body Mass Index (BMI) is commonly used to evaluate obesity in clinical practice, and obesity is defined when BMI exceeds 28 kg/m2. Overweight and obesity have continued to rise worldwide in recent years. According to the World Health Organization, by 2015, approximately 2.3 billion adults will be overweight, and more than 700 million will be obese [1]. Overweight and obesity among children are also on the rise in China. In 2014, compared with 1985, the overweight rate of children over seven years old increased by 10%, and the obesity rate increased by 7%. It also shows that if effective intervention measures are not taken, by 2030, the detection rate of overweight and obesity in school-age children over seven years old will reach 28.0%, and the number will increase to 49.48 million [2]. However, according to statistics, there has been no significant social effect in curbing the rate of overweight in the past 15 years [3]. It shows that many key risk factors can be changed by individual or group efforts and concerted national and even global action.
Globally, obesity has become a public safety problem. The increasing prevalence of obesity poses a considerable threat to public health, leading to complications such as type 2 diabetes mellitus, cardiovascular disease, and metabolic disorder syndrome [4-7]. In 2015, statistical data showed that its complications caused 4 million deaths worldwide in the past decade [8]. Therefore, the prevention and treatment of obesity are crucial. In recent years, scholars have found that obesity is associated with periodontitis and proposed that inflammatory cell markers, adipocytokines, oxidative stress, insulin resistance, and microbiota can be used to predict obesity in the early stage [9,10], which provides new research ideas for the early prevention and control of obesity. At the same time, researchers have found that inflammatory cell markers, adipocytokines, oxidative stress, insulin resistance, and microbiota in obese people also play important roles in developing periodontitis.
Periodontitis
Periodontitis is a chronic inflammatory disease caused by multiple factors resulting from a complex dynamic interaction among specific bacterial pathogens, destructive host immune responses, and environmental factors (such as smoking) [11]. Its main feature is the progressive destruction of tooth-supporting tissues. It is characterized by gingival bleeding, clinical attachment loss, periodontal pocket formation, alveolar bone loss assessed by imaging, pathological tooth displacement, and loosening [12]. In general, oral examination and imaging examination are necessary for clinical diagnosis.
The initiating factor of periodontitis is dental plaque, a kind of biofilm attached to the tooth surface. It has rich bacterial diversity and complex structure with a high density of bacteria, which not only helps protect the bacteria against external adverse factors but also promotes the synergistic interaction between bacteria and the formation of a quorum sensing system. Thus, they have higher virulence and environmental adaptability [13]. Existing studies have fully demonstrated that Aggregatibacter actinomycetemcomitans (A.a) and Porphyromonasgingivalis (P.g) in dental plaque play an important role in the occurrence and development of periodontitis [14,15].
Correlation between Obesity and Periodontitis
A cross-sectional study found that the prevalence of periodontitis in grade 1, 2, and 3 obesity was 79.2%, 2.8%, and 1.6%, respectively. Periodontal pocket depth was significantly correlated with the determinants of obesity, especially in grade 2 and 3 obese individuals [16], indicating that the higher the obesity grade, the higher the prevalence of periodontitis. It may be related to many important factors in obesity. Maulani et al. [17] analyzed the BMI and periodontitis related indexes of 262 subjects. They found that people with BMI greater than 25 kg/m2 were more correlated with periodontitis-related indexes and had a higher risk of periodontitis than normal-weight people. These results suggest that obesity plays a role in the pathogenesis of periodontitis. In addition, complications of obesity have also been found to be associated with the development of periodontitis. Mukherjee et al. [18] conducted a cross-sectional study on 250 subjects and found that hypertension and BMI were risk factors for periodontitis. A moderate positive correlation was found between periodontitis and obstructive sleep apnea. In obese people with Insulin Resistance (IR), the probability of periodontitis is greater but not more serious [19]. Oliveira et al. [20] found that obese patients with metabolic syndrome or diabetes had an increased risk of periimplantitis. These results suggest that complications of obesity also increase the risk of periodontitis.
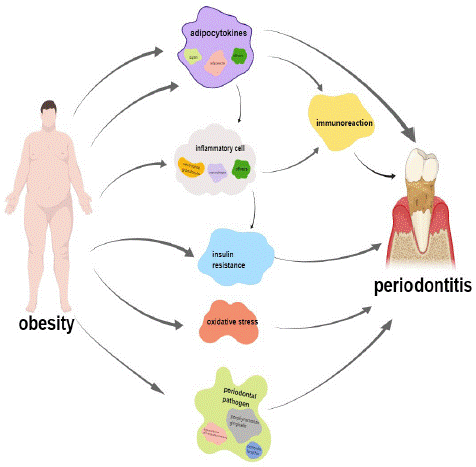
Figure 1: Possible ways that obesity promotes the development of periodontitis.
Numerous studies have shown that obese people have higher levels of periodontal pathogens. Through checkerboard DNA-DNA hybridization, Maciel et al. [21] found that obese people, regardless of the presence of periodontitis, showed higher levels of periodontal pathogens than normal-weight people, especially in patients with chronic periodontitis. Andrade et al. [22] analyzed the subgingival biofilms of overweight and obese young people without damaging periodontal diseases. They found that the level of P.g in overweight and obese people was significantly higher than in the normal weight group. There was a significant positive correlation between BMI and Prevotella, Lactobacillus, and actinomycetes. Ebersole et al. [23] performed ELISA on healthy subjects, gingivitis patients, and periodontitis patients and found that the levels of multiple IgG subclasses of periodontal pathogens were significantly reduced in obese subjects. In addition, Balakrishnan et al. [24] found through 16S rDNA sequencing technology that obesity in African children was related to oral microbiota, especially with A.a, revealing that oral microbiota may be related to race and obesity. These results indicate that obese people have higher levels of periodontal pathogens, indicating a greater tendency to develop periodontitis and suggesting that specific oral microbial species have the potential to be one of the targets for the diagnosis and treatment of obesity.
Factors of Obesity Affecting Periodontitis
Adipokines
Obesity is a state of chronic systemic inflammation. Adipocytes can secrete pro-inflammatory and anti-inflammatory adipokines such as leptin, visfatin, and adiponectin. When the pro-inflammatory factors and anti-inflammatory factors in the body are unbalanced, the body will have a chronic inflammatory state [25].
Adiponectin is an anti-inflammatory adipokine secreted by white adipose tissue [26], which plays an important role in inhibiting pathogenic microorganisms in the pathogenesis of periodontitis. LPS from P.g can stimulate the secretion of IL-6 and MMP-1 by gingival fibroblasts, and the expression level of MMP-1 increases with the worsening of periodontal inflammation, which is one of the key enzymes leading to alveolar bone resorption [27]. Adiponectin can inhibit the function of P.g and has anti-inflammatory, anti-bone resorption and matrix degradation effects [28]. Obese patients are often accompanied by hypoadiponectin, which weakens or even loses the anti-inflammatory effect of adiponectin [29], further revealing that obese people tend to suffer from periodontitis. Leptin, also known as an anti-obesity factor, acts on the arcuate nucleus of the hypothalamus by binding to leptin receptors and plays a role in regulating appetite and body weight [30]. Elevated levels of leptin in obese people do not suppress appetite, known as leptin resistance, and leptin resistance is one of the characteristics that contribute to the pathology of obesity. Leptin can regulate signalling pathways that affect the body's immune function [31], It can signal the formation of blood cells and lymphocyte proliferation, activation of mononuclear phagocytes, dendritic cells, and natural killer cells, regulate the adaptability of T and B cell-mediated immune response, promote the synthesis of cytokines, and stimulate the inflammatory reaction [32].
Inflammatory Reaction
The content of macrophages in adipose tissue of obese people is significantly increased, and the polarization phenotype is transformed from anti-inflammatory M2 type to pro-inflammatory M1 type, which can secrete IL-6, TNF-a, IL-1β, and promote the formation of periodontitis [33]. High concentrations of TNF-a secreted by macrophages can promote the synthesis of degrading enzymes by stimulating fibroblasts and activating osteoclasts to promote bone resorption, thus aggravating the development of periodontitis. On the other hand, a high concentration of TNF-a promotes the expression of osteoclast genes, such as receptor activator ligand of nuclear factor-κB, mainly through the nuclear factor-κB pathway, which promotes the maturation and differentiation of osteoclasts and causes bone resorption [34]. Il-6 is a multifunctional cytokine which can induce the synthesis of C-reactive protein in the liver, activate neutrophils, produce a condensation effect on inflammatory cells, promote the activation and release of inflammatory mediators, and lead to the aggravation of periodontal inflammatory reaction [35].
Immune Response
The adipokines produced by adipocytes and the TNF-a, IL-6, IL-1β and MMP-1 produced by inflammatory cells will inevitably cause the body's immune response. Many immune cells (such as macrophages, neutrophils, etc.) will infiltrate the body or the local area. After the immune cells are activated, they release inflammatory factors (such as TNF-a, IL-6, Il-1β, etc.), leading to aggravation of inflammatory reaction [36]. On the other hand, the immune response that occurs when the body prevents microbial invasion or diffusion will also damage local periodontal tissues [37].
Oxidative Stress
The chronic inflammatory state of obesity is closely related to oxidative stress. Studies have shown that the knockdown of the Toll-Like Receptor (TLR) 2 gene in obese mice inhibited the high expression of MyD88, p38MAPK, IL-6 mRNA and TNF-a mRNA and alleviated the oxidative stress state of adipose tissue [38], suggesting that high Free Fatty Acids (FFA) can activate TLR2. Furthermore, the MyD88-dependent p38MAPK pathway can activate downstream inflammatory factors and put adipose tissue under oxidative stress. Inflammatory factors play a significant role in periodontitis, but at the same time, oxidative stress also promotes the pathogenesis of periodontitis.
Reactive Oxygen Species (ROS) are the main substances that induce oxidative stress in the body. Under normal circumstances, ROS at the physiological level can regulate the homeostasis, signal transduction, apoptosis and other physiological activities of periodontal cells. On the other hand, excessive ROS exert cytotoxicity, interfere with the growth and cell cycle progression of human gingival fibroblasts, induces apoptosis of gingival fibroblasts, and induces periodontitis [39]. The evidence suggests that osteoclast formation occurs in ROS, where RANKL interactions trigger signalling cascades. Furthermore, the cascade causes the up-regulation of osteoclast genes, such as Tartrate-Tolerant Acid Phosphatase (TRAP) and histone K, which is the leading cause of alveolar bone loss and eventual tooth loss in periodontal disease [40]. The result of oxidative stress on periodontal destruction is bone loss, and one of the main features of periodontitis is alveolar bone loss; the final results of both are consistent.
Insulin Resistance
Insulin is also an important factor in aggravating periodontitis. Patients with insulin resistance without abdominal obesity are more likely to suffer from severe periodontitis. In normal weight people with abdominal obesity, insulin resistance can be considered as an independent risk factor for periodontal disease [41]. Fatty acid-free foods lead to not only obesity but also insulin resistance through the disappearance of acceptable pancreatic β-cells [42]. Proinflammatory cytokines secreted by adipose tissue, such as IL-6, IL-1 and TNF-a, can induce the release of acute phase reactants such as CRP and fibrinogen from the liver, thereby amplifying the existing inflammatory response and promoting insulin resistance [43]. In turn, insulin resistance can lead to generalized hyperinflammatory states, including periodontitis [42].
Changes in the Increase of Periodontal Pathogens Caused by Obesity
Dental plaque biofilm is the initiating factor of periodontitis. Once the biofilm is formed, bacteria in the mouth begin to colonize, among which P.g, A.a, and Forsetanella have been proven to be one of the well-documented bacterial communities in the occurrence of periodontitis. During pathogen community formation, key pathogens modulate immune responses and disrupt host immune surveillance, shifting the balance from homeostasis to imbalance. Pathogenic bacteria can also promote morbidity by enhancing direct interactions between oral pathogens. Virulence factors are molecules expressed by an organism at different stages of its life cycle that can damage the host. For example, Aa can produce leukotoxin, bacteriocin, chemoattractant, endotoxin, and bone resorption factor [44]. The outer membrane vesicles of P.g can communicate with host cells and other microorganisms and transmit virulence factors such as gingival and collagenase, thus causing changes in host cells [45]. Periodontal pathogens have two kinds of direct and indirect effects on the pathogenesis of periodontitis. The former is mainly when the microorganisms colonize and propagate in the periodontal tissue, invade the host tissue, escape the host defense function, and finally damage the periodontal tissue. The latter is the body's immune response to microorganisms and their toxic products; the immune response occurs when the body prevents microorganisms from invading or spreading and damaging the local periodontal tissue.
Other Factors
Not only does obesity itself harm periodontal health, but its complications (such as metabolic syndrome and diabetes) also have an impact on periodontal health. Implants are the third set of human teeth, and their successful osseointegration depends on the wound-healing mechanism. Recent studies have reported that systemic pro-inflammatory conditions and altered immune and microbiome responses in patients with hyperglycemia influence catabolic and anabolic events in bone healing, including increased osteoclast production and impaired osteoblast activity; In addition, chronic hyperglycemia and associated micro vascular and macro vascular diseases lead to delayed wound healing [20], which is a concern for tissue healing of implants. In addition, high glucose levels can stimulate the production of more free radicals and reduce the clearance of ROS in the body through multiple pathways, leading to oxidative stress. ROS damage is non-specific, and periodontal tissue may also be damaged [46].
Summary
Obesity is becoming a major global public health security problem. With the continuous improvement of people’s living standards, people’s lifestyles are gradually changing, such as the popularity of high-calorie food and fast food, transportation tools, and the influx of electronic products, so that the energy intake is far greater than the energy consumption, leading to the continuous rise of obesity rate. It has also led to increased cardiovascular disease, type 2 diabetes, metabolic disorders, and cancer. With the continuous update of research technology, scholars have found that obesity and periodontitis influence and promote each other.
This review focuses on the obesity-promoting effect of periodontitis. First, the obese state of chronic inflammation promotes the occurrence and development of periodontitis. Secondly, obesity directly or indirectly leads to the body’s resistance to oxidative stress or insulin, to a certain extent, promoting periodontitis. In addition, the study found that the obese group contained higher levels of periodontal pathogens. It provides more initiating factors for the occurrence of periodontitis. Therefore, obesity is one of the risk factors for periodontitis. Therefore, if obesity can be controlled with time and early intervention, it not only has an excellent therapeutic effect on obesity and obesity complications but also can prevent the occurrence of periodontitis to a certain extent.
Funding
This work was supported by the Longyuan Youth Innovation and Entrepreneurship Talents (Team Project) (2022LQTD57), Lanzhou Chengguan District Science and Technology Plan Project (2019RCCX0033), Lanzhou University Special Research Project of Serving the Economic and Social Development of Gansu Province (2019-FWZX-03).
Author Contributions
Yuqin Yan participated in the conception and design of the study, manuscript writing, and revision. Wei Wang and Fanrong Yu participated in the data collection and revision. Shaochen Su designed the study and contributed to the introduction and the discussion. All authors read and approved the final manuscript.
References
- Bascifici FA, Karaman. Effects of a modified acrylic bonded rapid maxillary expansion appliance and vertical chin cap on dentofacial structures. Angle Orthod. 2002; 72: 61-71.
- Burke M, Jacobson A. Vertical changes in high-angle Class II division 1 patients treated with cervical or occipital pull headgear. Am J Orthod Dentofacial Orthop. 1992; 102: 501-508.
- Cangialosi TJ. Skeletal morphologic features of anterior open bite. Am J Orthod Dentofacial Orthop. 2003; 85: 28–36.
- Carvalho AC, Paiva SM, Scarpelli AC, Viegas CM, Ferreira FM, Pordeus AI. Prevalence of malocclusion in primary dentition in a population-based sample of Brazilian preschool children. Eur J Paediatric Dent. 2011; 12: 107-111.
- Cerruto C, Cozzani P, Cozzani M. Compliance-free and non-invasive treatment of an anterior open bite in a 11-year-old girl. Eur J Paediatric Dent. 2018; 19: 282-286.
- Gracco A, Spena R. Vertical control in nonextraction treatment of growing patients with anterior skeletal open bite. J Clin Orthod. 2008; 42: 443-9.
- Proffit WR, Fields HW. Occlusal forces in normal and long face children. J Dental Res. 1983; 62: 571-4.
- Urzal V, Braga AC, Ferreira AP. Oral habits as risk factors for anterior open bite in the deciduous and mixed dentition – cross-sectional study. Eur J Paediatric Dent. 2013; 14: 299-302.
- Wise J, Magness WB, Powers J. Maxillary molar vertical control with the use of transpalatal arches. Am J Orthod Dentofacial Orthop. 1994; 106: 403-8.