
Review Article
Austin J Dent. 2015;2(2): 1017.
Microbial Colonization of Conventional and Enhanced Elastomeric Chains in Orthodontic Patients
Mattos CT1, Baratieri C2, Freitas AOA3, Alviano DS4, Souza MMG3, Araújo MTS3, Nojima LI3, Nojima MCG3*
1Dental Clinics Department, Universidade Federal Fluminense, Brazil
2Department of Orthodontics, Universidade Federal de Santa Catarina, Brazil
3Department of Pediatric Dentistry and Orthodontics, Universidade Federal do Rio de Janeiro, Brazil
4Paulo de Góes Institute of Microbiology, Universidade Federal do Rio de Janeiro, Brazil
*Corresponding author: Nojima MCG, Departamento de Ortodontia, Faculdade de Odontologia, Universidade Federal do Rio de Janeiro, Av. Professor Rodolpho Paulo Rocco, 325, Ilha do Fundão, Rio de Janeiro, RJ, CEP: 21941-617, Brazil
Received: September 25, 2014; Accepted: February 23, 2015; Published: February 25, 2015
Abstract
The aim of this study was to evaluate microbial contamination of enhanced elastomeric chain (Super Slick, Metafisica technology, TP Orthodontics) compared to a conventional chain in situ. Twenty-six segments of elastomeric chains were placed in thirteen patients attached to their archwires. After 21 days, the chains were retrieved and their biological material was submitted to microbiological processing and cultured in nonspecific media and in media specific to detect Streptococcus spp, Lactobacilli spp and Candida spp. The number of Colony Forming Units (CFU) was macroscopically counted. Elastomeric chains were weighed for quantification of the mass of accumulated plaque. The results of colony counting were described with frequencies and analyzed with the Wilcoxon test. Statistical analysis showed no differences between the chains in number of CFUs on any medium tested. Streptococcus spp were observed on 61.5% of conventional chains and on 41.7% of Super Slick chains. The mean difference between the initial and final weight of the chains was 0.0030 g for conventional chains and 0.0031 g for Super Slick chains. The elastomeric chains had similar bacterial colonization and plaque accumulation properties. Further investigations are needed before these modified chains be considered an accessory with less bacterial biofilm formation.
Key words: Orthodontics; Microbiology; Elastomers
Introduction
The placement of orthodontic appliances increases dental biofilm accumulation [1] and favors Streptococcus mutans colonization [2]. The adoption of strict measures for maintenance of oral health during orthodontic therapy has become more justifiable in view of current concepts of health promotion and biosecurity.
The aim of orthodontic bacterial adhesion research is to prevent the formation of plaque on orthodontic materials [3]. Orthodontic materials with modified properties that are claimed to provide greater efficacy in controlling bacterial development on their surfaces have been introduced [4].
Elastomeric chains are widely used in Orthodontics, especially in space closure, in the retraction of anterior teeth and in the correction of rotations. However, they can act as a potential host for microbial accumulation [5].
Coating elastomeric chains with a hydrophobic polymeric substance has been suggested as a methodology in decreasing friction at the archwire-bracket interface and in repelling salivary adherends. They also provide good retention, tensile strength and better elastic properties [5]. The Metafasix technology (TP Orthodontics, LaPorte, Ind), used in the Super Slick elastomeric chain (TP Orthodontics, LaPorte, Ind), is a water-insoluble, hidrogel-polymer coating that transforms the polyurethane-based elastomeric surface into a highly smooth surface when moistened. As a consequence, less accumulation of bacterial biofilm on the chain surface during orthodontic therapy is expected [4].
There are no published studies assessing microbial contamination on the surface of elastomeric chains modified by Metafasix technology compared to conventional elastomeric chains submitted to the oral environment.
Therefore the aim of the present study was to evaluate the microbial contamination in situ of Super Slick elastomeric chain compared to a conventional chain exposed to the oral environment for 21 days. The null hypothesis raised is that there would be no difference in microbial contamination of Super Slick elastomeric chain and the conventional one.
Material and Methods
A sample size calculation was made in order to detect a significant difference with a power value of 80% and a significance level of 5%, which indicated twelve patients, according to the study design adopted.
Thirteen patients of both genders, aged 17 to 30 years, who were undergoing orthodontic treatment with fixed standard edgewise appliance in the finishing stage and with rectangular archwires in both arches, were selected from the orthodontic clinic of the Post-graduation course of the Faculty of Dentistry of the Federal University of Rio de Janeiro. Individuals who had any systemic disease or periodontal disease, patients who had used antibiotics within the previous three months and in current use of antimicrobial mouthwashes were excluded from the study. This research project was approved by the local Ethics in Research Committee and written informed consent was obtained from the patients or their respective parents.
In each volunteer, two quadrants of the mouth were randomly chosen using random number tables. The generator of the tables and the researcher who placed the chains were different individuals (CB and CTM). Two crimpable hooks 18 mm apart from each other were attached to the archwires in the interbracket region in the quadrants selected. A Super Slick elastomeric chain (size, mini; clear color; TP Orthodontics, LaPorte, Ind, USA) was stretched in one quadrant supported by the hooks and an aesthetic conventional elastomeric chain (size, short; pearl color; American Orthodontics, Sheboygan, Wis, USA) was stretched in the other quadrant chosen. Both elastomeric chains used had an equal number of five loops. A pilot study showed that the use of five loops of these two specific elastomeric chains of the size mentioned in the specified distance produced a similar force. The mean force produced by the Super Slick elastomeric chain was 176.85 g and the mean force produced by the conventional chain was 171.54 g. The force was measured on the EMIC DL 10000 universal testing machine (São José dos Pinhais, Brazil).
The volunteers were oriented on oral hygiene performance. They were supplied with the same toothbrush (Colgate Professional Extra Clean, Colgate-Palmolive, São Paulo, Brazil) and toothpaste (Cogate Total 12, Colgate-Palmolive, São Paulo, Brazil), and asked to use them for oral hygiene during the period of the trial and to refrain from any other oral hygiene products. The volunteers were also asked to maintain the dietary habits recommended by the clinic of the Postgraduate Course in Orthodontics.
The biological material was collected and processed by one trained and calibrated researcher, who performed every microbial test. After 21 days, the elastomeric chains were carefully removed and stored individually in Eppendorf tubes. Each tube containing an elastomeric chain was weighed on high precision scales (Shimadzu, São Paulo, and São Paulo). One milliliter of sterile saline (0.85% sodium chloride) was added in each tube so that the material could be used for subsequent cell culture. The interval between collection and transportation of material for cell culture was less than 2 hours. The tubes containing the biological material were vortexed for 30 seconds. The resulting suspension was further diluted to 10-2 and 10-3 in sterile saline. Solid culture media were used for nonspecific analysis (Brain Heart Infusion – BHI) and for specific analysis to detect Streptococcus spp (Mitis salivarius), Lactobacilli spp (Rogosa) and Candida spp (CHROMagar). The biological material was inoculated onto Petri plates. The 10-3 dilution was used on the BHI and on the Mitis salivarius media and the 10-2 dilution on the Rogosa and CHROMagar media. These dilutions were chosen based on a pilot study. Streptococcus spp incubation was done with oxygen restriction and nonspecific, Lactobacilli spp and Candida spp were cultured without oxygen restriction. The Petri dishes were maintained at 37° C inside a heater for 24 hours, except for the CHROMagar medium, which was kept in the heater for 48 hours to allow microbial growth. The identification and counting of colonies were done macroscopically. The number of viable microorganisms was calculated from the number of Colony Forming Units (CFU).
After cell culture procedures, the elastomeric chains were washed three times inside the Eppendorf tubes with Phosphate Buffered Saline Solution (PBS) and the tubes were stored in a heater at 37° C with the lid open for 24 hours to allow them to dry. Then the tubes containing the chains were weighed again in order to compare plaque accumulation on the elastomeric chains. The difference between the two weighings was computed for each elastomeric chain in order to quantify the mass of the plaque accumulated on the chains. The same blind researcher did all microbiological procedures.
The results of colony counting were described with frequencies and analyzed statistically with the Wilcoxon nonparametric test at the 5% significance level. All statistical analyses were performed using GraphPad Prism statistical software for Windows (version 4.0, GraphPad Software, San Diego, Calif).
Results
One Super Slick chain of one patient had been lost when she came for the second visit in order to have the elastomeric chains removed.
Microbial analyses showed nonspecific microorganisms on 60% of all elastomeric chains. Their presence was found on 69.3% of the conventional chains and on 49.8% of Super Slick chains. Streptococcus spp were observed on 52% of all elastomeric chains, more specifically on 61.6% of the conventional chains and on 41.6% of Super Slick chains. Lactobacilli spp were not found on any chains. One colonyforming unit of Candida spp was observed on two conventional chains and in one Super Slick chain. Table 1 shows the frequency in valid percent of the number of CFU for each media tested. Figure 1 shows the comparison between conventional and Super Slick elastomeric chains in the distribution of CFUs counted on the BHI and on the Mitis salivarius media.
CFUs
Nonspecific
Streptococcus spp
Lactobacilli spp
Candida spp
conventional
Super Slick
P-value*
conventional
Super Slick
P-value*
conventional
Super Slick
P-value*
conventional
Super Slick
P-value*
0
30.7%
50.2%
0.368
38.4%
58.4%
0.453
100.0%
100.0%
1.00
84.6%
91.7%
0.595
1-10
38.5%
24.9%
46.2%
25.0%
0.0%
0.0%
15.4%
8.3%
11-50
30.8%
16.6%
7.7%
8.3%
0.0%
0.0%
0.0%
0.0%
51-100
0.0%
8.3%
7.7%
8.3%
0.0%
0.0%
0.0%
0.0%
100%
100%
100%
100%
100%
100%
100%
100%
Table 1: Frequency of colony forming units (CFUs) in valid percent (%) of different microorganisms in conventional and Super Slick elastomeric chains exposed to the oral environment for 21 days. The 10-3 dilution was used on the BHI and on the Mitis salivarius media and the 10-2 dilution on the Rogosa and CHROMagar media.
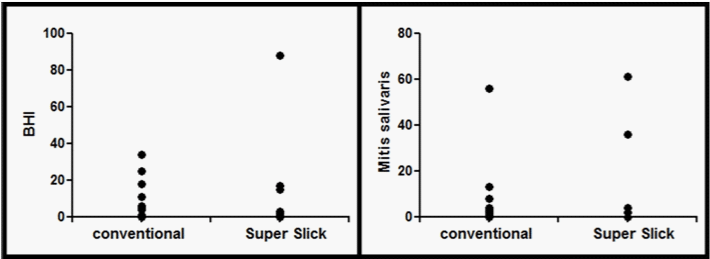
Figure 1: Comparison between the conventional and the Super Slick elastomeric chains in the distribution of CFUs counted on the BHI and on the Mitis salivarius
media (10-2 dilution).
Statistical analysis by the Wilcoxon nonparametric test showed no significant differences (p>0.05) between the conventional and the Super Slick elastomeric chains in the number of CFUs on any medium tested.
The mean difference between the weight of the elastomeric chains before and after being rinsed was 0.0030 g (standard deviation of 0.0014 g) for the conventional chains and 0.0031 g (standard deviation of 0.0016) for the Super Slick chains.
Discussion
The null hypothesis tested in this study that there would be no difference in microbial colonization of Super Slick elastomeric chain and the conventional one was confirmed by the results.
The prevention of enamel decalcification during fixed appliance treatment remains a challenge to the orthodontist [6-7]. Since individuals who undergo orthodontic treatment have elevated levels of Streptococcus mutans [8], the control of plaque is fundamental in the prevention of caries and periodontitis [9]. Unless patients receive specific instructions on appropriate home care, abundant plaque may form on bonded teeth within one week [10]. In addition to brackets and orthodontic bands, other accessories can lead to the accumulation of microorganisms. Each material can induce specific alterations in the oral environment, such as pH reduction and the prolonged accumulation of dental biofilm [11]. Elastomeric rings may lead to an increased retention of plaque and risk of gingival bleeding compared to steel ligatures [1]. Türkkahraman et al. [12] also observed that gingival tissue adjacent to teeth ligated with elastomeric rings was more prone to bleeding. Steinberg and Eyal [13] observed that modules have demonstrated high adsorption capability of whole saliva protein constituents, high adhesion capability to albumin and amylase as well as high affinity toward S. sobrinus.
The use of elastomeric chains may introduce broader retention areas for plaque accumulation. And the plaque accumulated on the exposed material may have detrimental effects on the surrounding hard and soft tissues, since its proximity to bracket margins may enhance the possibility of undesirable effects such as enamel decalcification or gingival inflammation [14]. It is reasonable then that this important subject be aim of research and become the concern of manufacturers of orthodontic accessories.
Information concerning initial bacterial adhesion to the surfaces of orthodontic materials has been provided by previous studies and could be used in the development of orthodontic biomaterials with enhanced properties [15,16]. The Super Slick elastomeric chains, produced with the Metafasix technology, are expected to bear less biofilm accumulation than conventional chains.
Casaccia et al. [17] showed the absence of biological contamination on packed elastomeric chains from different manufacturers due to their manufacturing process, even though they do not inhibit bacterial or fungal growth on their surfaces. This potentially reduces the bias of contamination of elastomeric chains prior to their insertion in the mouth.
In this study there was a concern about standardization of the force applied by the elastomeric chains. That was considered important in a comparison of two types of chains to reduce bias and to better simulate clinical conditions. Elastomerics are affected by moisture and the amount of stretching influences directly on permanent deformation and force decrease [18,19]. Special care was also taken in the randomization of the location of the chains in the oral environment to avoid bias related to different hygiene control individual patterns.
The 10-2 dilution was used on the Rogosa and CHROMagar media as it permitted counting of CFUs in the pilot study. However, when this dilution was used on the BHI and on the Mitis salivarius media, the CFUs were uncountable. The 10-3 dilution was then adopted for the BHI and Mitis salivarius media and it proved to be adequate in the circumstances of this research.
Only one study [4] assessed the microbial contamination of Super Slick elastomerics in orthodontic patients. This study, however evaluated Super Slick elastomeric rings. The results of the study of Magno et al. [4] showed that, after a 15-day intraoral period, S. mutans colonies or biofilms were observed on the entire surface of 85% of the Super Slick elastomeric rings by a ranked scale analysis. The contamination of Super Slick ligatures was significantly greater than that of conventional ligatures. In the present study, Streptococcus spp were observed only on 41.7% of the Super Slick elastomeric chains, whereas 61.5% of conventional chains showed contamination by the same genus. The differences between our results and the results of Magno et al. [4] may be due to methodological differences or to differences in the material observed (elastomeric chains and elastomeric rings, respectively).
Nonetheless, the statistical test applied showed no significant differences between the conventional and the Super Slick elastomeric chains in the number of CFUs on any medium tested. This may be due to the great variance observed in the number of CFUs counted on the BHI and on the Mitis salivarius media, which could be partially explained by individual differences in oral plaque composition.
The mean difference between the weight of the elastomeric chains before and after being rinsed may be attributed to the plaque accumulated on the chains. This difference was very similar on both types of chain used in the study, which suggests that biofilm accumulation was alike.
This study used cell culture in order to conduct a preliminary analysis of microbial accumulation on the investigated elastomeric chains. New studies using specific and unspecific PCRs should be performed for further information.
Conclusion
According to the methodology and based on the results of this study, the following conclusions can be drawn: regardless of the material, elastomeric chains have similar bacterial colonization and plaque accumulation properties; further investigations are needed before modified elastomeric chains be advised for clinical use as an orthodontic accessory with less bacterial biofilm formation.
Acknowledgement
The authors declare no conflict of interest. The authors thank CAPES (Coordination for Improvement of Higher Level – or Education – Personnel) for scholarship during the PhD program.
References
- Alves de Souza R, Borges de Araujo Magnani MB, Nouer DF, Oliveira da Silva C, Klein MI, Sallum EA, et al. Periodontal and microbiologic evaluation of 2 methods of archwire ligation: Ligature wires and elastomeric rings. Am J Orthod Dentofacial Orthop. 2008; 134: 506-512.
- Mattingly JA, Sauer GJ, Yancey JM, Arnold RR. Enhancement of Streptococcus mutans colonization by direct bonded orthodontic appliances. J Dent Res. 1983; 62: 1209-1211.
- Faltermeier A, Bürgers R, Rosentritt M. Bacterial adhesion of Streptococcus mutans to esthetic bracket materials. Am J Orthod Dentofacial Orthop. 2008; 133: 99-103.
- Magno AF, Enoki C, Ito IY, Matsumoto MA, Faria G, Nelson-Filho P. In-vivo evaluation of the contamination of Super Slick elastomeric rings by Streptococcus mutans in orthodontic patients. Am J Orthod Dentofacial Orthop. 2008; 133: S104-109.
- Leander D, Kumar JK. Comparative evaluation of frictional characteristics of coated low friction ligatures - Super Slick Ties with conventional uncoated ligatures. Indian J Dent Res. 2011; 22: 90-94.
- Banks PA, Chadwick SM, Asher-McDade C, Wright JL. Fluoride-releasing elastomerics--a prospective controlled clinical trial. Eur J Orthod. 2000; 22: 401-407.
- Wilson TG, Gregory RL. Clinical effectiveness of fluoride-releasing elastomers. I: Salivary Streptococcus mutans numbers. Am J Orthod Dentofacial Orthop. 1995; 107: 293-297.
- Rosenbloom RG, Tinanoff N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991; 100: 35-37.
- Benson PE, Douglas CW, Martin MV. Fluoridated elastomers: effect on the microbiology of plaque. Am J Orthod Dentofacial Orthop. 2004; 126: 325-330.
- Sukontapatipark W, el-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001; 23: 475-484.
- Bretas SM, Macari S, Elias AM, Ito IY, Matsumoto MA. Effect of 0.4% stannous fluoride gel on Streptococci mutans in relation to elastomeric rings and steel ligatures in orthodontic patients. Am J Orthod Dentofacial Orthop. 2005; 127: 428-433.
- Turkkahraman H, Sayin MO, Bozkurt FY, Yetkin Z, Kaya S, Onal S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005; 75: 231-236.
- Steinberg D, Eyal S. Initial biofilm formation of Streptococcus sobrinus on various orthodontics appliances. J Oral Rehabil. 2004; 31: 1041-1045.
- Eliades T, Eliades G, Watts DC. Structural conformation of in vitro and in vivo aged orthodontic elastomeric modules. Eur J Orthod. 1999; 21: 649-658.
- Lee SJ, Kho HS, Lee SW, Yang WS. Experimental salivary pellicles on the surface of orthodontic materials. Am J Orthod Dentofacial Orthop. 2001; 119: 59-66.
- Akgun OM, Altug H, Karacay S, Polat GG, Duyan S, Bedir O. Effect of 2 elastomeric ligatures on microbial flora and periodontal status in orthodontic patients. Am J Orthod Dentofacial Orthop. 2014; 145: 667-671.
- Rembowski Casaccia G, Gomes JC, Alviano DS, de Oliveira Ruellas AC, Sant' Anna EF. Microbiological evaluation of elastomeric chains. Angle Orthod. 2007; 77: 890-893.
- Taloumis LJ, Smith TM, Hondrum SO, Lorton L. Force decay and deformation of orthodontic elastomeric ligatures. Am J Orthod Dentofacial Orthop. 1997; 111: 1-11.
- Baratieri C, Mattos CT, Alves M, Lau TC, Nojima LI, de Souza MM, et al. In situ evaluation of orthodontic elastomeric chains. Braz Dent J. 2012; 23: 394-398.