
Research Article
Austin J Dent. 2015; 2(3): 1024.
Histomorphometric and Biochemical Evaluations of Stem Cells for Osteogenesis and Neogenesis
Alkaisi Amera*
Department of Oral and Maxillofacial Surgery, University of Anbar, Iraq
*Corresponding author: Alkaisi Amera, Department of Oral and Maxillofacial Surgery, University of Anbar, Iraq
Received: July 02, 2015; Accepted: September 01, 2015; Published: September 10, 2015
Abstract
The purpose of this study was to quantify bone tissue and blood vessel area in the central part of regenerate after transplantation of Stem Cells From Human Exfoliated Deciduous teeth (SHED) in rabbit Mandibular Distraction Osteo Genesis (MDO) in comparison to conventional MDO, As well to measure serum Alkaline Phosphatase (ALP) level and to correlate the 3 parameters. A randomized controlled trial was carried out with 18, 3 - 5.5 month old male New Zealand white rabbits, weighing 2.8 ± 0.2 kg. All animals were divided into 2 groups with 9 animal each (A, as experimental group and B, control). Six million cells were transplanted into the distracted area during the osteotomy period. After a 4-days latency period, a total of 6 mm was distracted for 6 days. The newly formed bone in the central area of the DO regenerates were analyzed histologically, histomorphometrically and biochemically at weeks 2, 4, and 6 postoperatively. All data were analyzed using non parametric Mann-Whitney U test. Spearman correlation test was used to correlate between the 3 variables. The significance was accepted when P is less than 0.05. More bone was observed in the central area of SHED transplanted group histologically and Histomorphologically, the percentage of newly formed bone after 2 weeks were 16 and 0; after 4 weeks, 39 and 22; and after 6 weeks, 27 and 23% in the SHED and control groups respectively. The difference between the groups was statistically significant (P = 0.024). The calculated percentage of blood vessels area at 2 weeks SHED and control group were 5.9 and 4, at 4 weeks 34.42 and 14.64 and at 6 weeks 17.64 and 19% respectively, however; the difference between the groups was statistically not significant P = 0.489. The mean difference of serum alkaline phosphatase level of SHED and control groups were 128.33 and 42.66 respectively with significant result, p< 0.001. A positive correlation was found between the volume density of bone and blood vessels P = 0.014, volume density of bone and ALP, P = 0.029 and no correlation between blood vessel volume and ALP level, p = 0.372.
Conclusion: The results of this study suggest that stem cell therapy accelerate callus and bone formation in mandibular osteodistraction, and increase blood vessels, therefore facilitated consolidation. It may be a valuable approach for clinical osteo distraction enhancement, especially in sites without enough osteogenic potential and vascularization.
Keywords: Distraction osteogenesis; Stem cells; Osteogenesis; Neogenesis
Introduction
Attention was directed toward mandibular Distraction Osteogenesis (DO), due to expanding demand for minimally invasive orofacial reconstructive techniques. Therefore, an adequate understanding of the biologic events, which take place during DO, is necessary to effectively modulate and manipulate the bony regeneration. The clinical goals of DO research include speedier formation of new bone, shorter fixation time, enhanced bone quality, and minimized risk of nonunion of the osteotomized edges. A multipotent postnatal stem cells have been identified, reside in the pulp of human primary (baby) teeth, these cells have been designated as SHED (Stem Cells from Human Exfoliated Deciduous teeth) [1]. They are isolated from a disposable source (i.e., exfoliated deciduous teeth) noninvasively and retain their potentiality after in vitro expansion, offer a significant advantages. SHED have been found to divide continuously and could be differentiated into a variety of other cell types including nerve, fat, bone, and tooth generating cells. Deciduous teeth may provide an ideal source of stem cells to repair damaged tooth structure, to regenerate bone and perhaps treat nerve damage. The use of SHED might bring advantages for tissue engineering over the use of stem cells from other sources as they have higher proliferation rate [1,2]. The use of multipotent postnatal stem cells from a disposable source that can be readily isolated noninvasively and retain their potentiality after in vitro expansion, offers a significant advantages. Importantly, these cells form a functional vasculature as well as connective tissue secreting cells of the soft and hard tissues of the tooth [3] however there are few studies regarding regeneration of bone defects using MSCs [4,5]. In DO, Kitoh et al. and Jiang et al. [6,7] found more new bone formation and faster calcification in the distracted callus in autologous BMMSCs injected distraction gap of the mandible when compared with the rabbits received saline injection. However very few researches found in the literature using SHED in bony defect and DO, de Mendonca Costa et al. [8] evaluated the capacity of human Dental Pulp Stem Cells (hDPSC), isolated from primary teeth to reconstruct a large-sized cranial bone defects in Non Immune Suppressed (NIS) rats, they found that hDPSC is another cell resource for correcting large cranial defects in rats and constitutes a promising model for reconstruction of human large cranial defects in craniofacial surgery to recruit murine host osteogenic cells [1,8]. Seo et al. [9] found that when SHED is transplanted into a critical-sized calvarial defect area, they generate bony tissue to repair the defect. However, only our previous study, Alkaisi et al. [10] was found using SHED in DO for the same purpose, therefore this study may be the 2nd one using SHED in DO to enhance bone formation. The utilization of DO in the craniofacial skeleton has been steadily increasing for a variety of reconstructive options [11-14] since its original discovery over a century ago by Codivilla [15]. Yet since its initial application for lengthening the mandible in congenital hypoplasia by McCarthy [16] in 1992, we still lack discrete, quantitative metrics to evaluate either the degree of success or failure of the technique. Establishing accurate and reproducible parameters to gauge Regenerate Healing (RG) during Mandibular Distraction Osteogenesis (MDO) should lead to advances in proper application of this technique and improved distraction protocols for enhanced clinical use. Experimental animal studies assessing regenerate healing in MDO using histological analysis have often been subjective and qualitative, or “semi” quantitative [17-19], lacking the measurable precision required to reproducibly determine outcomes. Few animal studies were found using histological measurements in their analysis [10,20, 21]. Several parameters have been used to evaluate regenerate progress include, percentage of new bone volume, cartilage and fibrous tissue, number of blood vessels, number of osteo blasts and osteocytes and others. Ethically it is difficult to apply same method of histomorphometric measurement in human; however there are 2 studies found in the literature measuring some histological parameters in vertical DO using a small biopsy [22,23].The need for non invasive technique to follow and evaluate the stage of regenerate maturation, will help both surgeon and patient to optimize the treatment. Alkaline Phosphatase (ALP), is the main glycosylated protein present in the bone, bound to osteo blast cell surfaces via a phosphoinositol linkage and found free within mineralized matrix [24] as well present in a soluble form in body fluids, including blood [25]. Four isoenzymes exist: intestinal, placental, germ cells and Tissue-Nonspecific, The Tissue-Nonspecific ALP (TNAP) has three isoform, expressed in bone, liver and kidney [26]. It is commonly used as a biochemical marker to assess osteo blast activity and plays as an undefined role in mineralization of bone [24]. Generally speaking, ALP expression increases during early osteogenesis, but when mineralization is advanced, its expression decrease; it is possible to assess Alkaline Phosphatase activity (ALP) expression and stain for matrix mineralization [27]. The purposes of this study were, to quantify bone healing in MDO with transplanted SHED compared to non transplanted one, using a 3, consolidation period [10], to quantitatively measure the bone tissue area in the Region of Interest (ROI), referred as Tissue Area (TA) within the central region of the regenerate after unilateral MDO+SHED in comparison to MDO, to measure blood vessel area and to correlate the amount of newly formed bone with the vessel area. A further specific aim was to measure serum ALP level and to correlate it with our two parameters used aiming to have a new non invasive parameter, can be applied clinically for human DO. We hypothesized that there are significantly higher bone formation, vascularization and serum ALP level in the SHED transplanted group than non transplanted one with significant positive correlation between the variables.
Materials and Methods
Animal care
Ethical approval was obtained from animal ethics committee Universti Sains Malaysia number: USM/Animal Ethics Approval/2010/ (58) (226). This experimental study was carried out with 18, 3 - 5.5 month old male New Zealand white rabbits, weighing 2.8 ± 0.2 kg. All animals were divided into 2 groups, 9 animal each (A as experimental group and B, control). Rabbits were housed in a pathogen-free separated cages with pellet and water.
Surgical procedure
General anesthesia was achieved via intramuscular injection of 35 mg/kg ketamine and xylazine (5 mg/kg). Local administration of 2% lidocaine (Astra, USA) at the surgical site was performed. Right mandible of the rabbits was exposed through a longitudinal incision on the inferior border. A vertical osteotomy was carried out between premolar and mental foramen and a custom-made internal with external activation part distraction device (A SUPER screw palatal expander (Ortho-Care UK Ltd), 12 +12 mm length stainless steel device with full distraction capacity of 12 mm after modification), was fixed to either side of the osteotomy cut and the surgical exposure was primarily closed in layers, the procedure performed according to Alkaisi et al. method [10]. The wound was cleaned with povidon iodine antiseptic and neomycin ointment (antibiotic) was applied.
Distraction osteogenesis protocol
Distraction osteogenesis protocol was 4 days latency period, distraction at a rate of 1 mm/day continued for 6 days resulting in a final lengthening of 6 mm. After the completion of distraction the animals were monitored at 2, 4 and 6 weeks post operatively for consolidation.
Stem cells preparation
SHED were isolated, cultured, characterized and expanded in vitro [10]. About 4-6, T75 tissue culture flasks 80-100% confluent were passaged, cells were counted and adjusted to 6×106. Group A, 6×106 SHED were transplanted in the osteotomy site (at osteotomy period) as an experimental and no transplantation were deposited in group B as a control.
Follow up
The animals were monitored at 2, 4 and 6 weeks period postoperatively. All animals were given an intramuscular injection of Baytril (Enrofloxacin) 10 mg/kg/day (Bayer, Shawnee Mission, KS, USA) once daily and Tramadol hydrochloric (UNICHEM-India), 2mg/kg once daily for pain relief for 7 days.
Histological examination
Each right hemimandible was dissected free of the surrounding tissue immersed in 4% formalin for 24 hours, decalcified in 10% solution of nitric acid for 4 days and automatically processed in a machine for dehydration then embedded in paraffin wax. Sections, 5μm in thickness were cut bucolingually with a microtome and stained with haematoxylin and eosin for light microscopy examination. New bone formation, blood vessels, and fibrous tissue in the central area were assessed.
Histomorphometric examination
All samples were analyzed histomorphometrically using Zeiss image analysis system (Carl Zeiss, MMI 0684-Germany). The images of the histological sections were scanned by the system and saved on a computer. Using computerized image analysis system software, Regions of Interest (ROI), area of 1mm² in the central area of the regenerate was designed, outlined and calculated in square micrometer (Figure 1). New bone trabeculaes outlined, measured and their percentage was calculated. Blood vessels were outlined, measured and the percentage of the vessel area was calculated (Figure 2). Vascular histomorphometry was performed according to Amir et al. [23] as follow: Pictures of the Region of Interest (ROI), were taken in which soft connective tissue and blood vessels were identified. The vascularization was quantified as follow: A blood vessel was defined as a lumen of various sizes, lined by elongated endothelial cells, sometimes surrounded by flattened or cuboidal smooth muscle cells. Occasionally, identification was facilitated by the presence of red blood cells in the lumen (Figure 3). Determination of the volume density of blood vessels defined as the percentage of total soft connective tissue area taken in by blood vessels. Blood vessel volume was calculated as [total blood vessel area/total soft tissue area] x 100%. The measurements were performed at 10 and 20xmagnification by two investigators (A.A and N.A) who were unaware of the origin of the sections. Of each biopsy, 3 sections at different depth were measured and the values averaged.
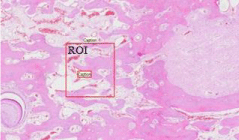
Figure 1: Designed Region of Interest (ROI), area of 1mm2 1X.
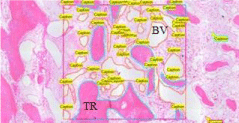
Figure 2: Outlining of Trabeculae (TR) and Blood Vessels (BV), 5X.
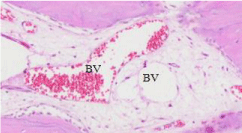
Figure 3: Identification of Blood Vessels (BV) 20X.
Biochemical evaluation
Samples of blood were taken from ear vein of the rabbit’s pre and 2 weeks post operatively. Blood samples were centrifuged and the serum transfer to the department of chemistry, School of Medical Sciences Universiti Sains Malaysia. The serum was analyzed for the level of serum alkaline phosphatase. All the procedures were carried out by one chemist that she did not know even the origin of the sample whether being from human or animal.
Statistical analysis
SPSS version 16 was utilized for data analysis as follows; The histomorphometric data representing percentage of bone area within the region of interest, blood vessels area, and alkaline phosphatase level, were analyzed using non parametric Mann-Whitney U test. Spearman correlation test was used to correlate between the 3 variables, new bone area, blood vessel area and difference of serum ALP level pre and postoperative 2 weeks. The significance was accepted when P is less than 0.05.
Results
Histological finding
A Progressive bone formation was observed in both groups starting at week 2 postoperatively till 6 weeks with higher bone formation in SHED group. In control group, at 2 weeks period fibrous tissue consisted of collagen fibers bundles were observed in the central area with minimal remaining hematoma present in most cases. There were no islands of new bone formed with reduced number of blood vessels. At 4 weeks more areas of bone formation were observed with thin and small bony trabeculae in loose fibrovascular stroma and moderate number of blood vessels. At 6 weeks there was more bone formation, however the amount were still little. In SHED groups there were higher bone formation than control starting at week two, trabiculae seen in the central area with highly vascular tissue, the amount increase highly at 4 and 6 weeks with complete bony union in the central area.
Bone histomorphometry
The histomorphometric analysis of the total amount of bone present within the central area of the defects revealed that, a higher amount of new bone formation was observed in SHED transplanted group than in control across at all time points. The semi-quantitative analysis of the woven bone produced in the central DO area revealed, the highest amount of newly produced woven bone was in the SHED group at 4 weeks (39%) and no bone trabiculi were observed in the central area at 2 weeks period in the control group. Histomorphometric measurement results using image analyzer were; 1) The total areas designed in the center of the distracted mandibles (Region of interest: ROI) were ranged between 1 to 1.03 mm². 2) The mean area of new bone volume were; at 2 weeks SHED and control groups 0.16 and 0, at 4 weeks 0.39 and 0.22 and at 6 weeks 0.27 and 0.23 mm² respectively (Table 1). 3) The calculated percentage of new bone area at 2 weeks SHED and control groups were 16 and 0, at 4 weeks 39 and 22 and, at 6 weeks 27 and 23% respectively (Figure 4), with P = 0.024, (Table 2).
Specimen
Area of new bone (mm2)
Area of new blood vessels (mm2)
SHED 2W
0.16
0.05
Control 2W
0
0.04
SHED 4W
0.39
0.21
Control 4W
0.22
013
SHED 6W
0.27
0.15
Control 6W
0.23
0.16
Table 1: Total area of newly formed bone and blood vessels in SHED and control.
Group
N
Median
Interquartile range
Min
Max
Z Statistic
P Value
SHED
9
37
24.20
13
46
-2.260
0.024
Control
9
19
23.50
0
24
Table 2: Comparing the percentage of bone volume between SHED and control groups.
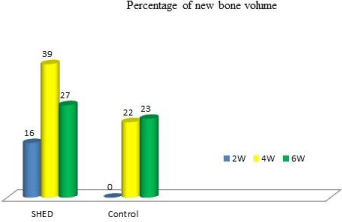
Figure 4: Percentage of new bone in the central area of DO regenerate (2W,2
weeks; 4W,4 weeks;6W,6 weeks).
Vascular histomorphometry
The mean area of vascular volume in SHED and control groups were 0.05 and 0.04 mm², at 4 weeks 0.21 and 0.13 and at 6 weeks 0.15 and 0.16 respectively (Table 1). The calculated percentage of blood vessels volume at 2 weeks SHED and control groups were 5.9 and 4, at 4 weeks 34.42 and 14.64 and at 6 weeks 17.64 and 19% respectively (Figure 5). The total area of the vascular bed (blood vessel volume density) in the central area of SHED group was higher than control, but the result was not significant P = 0.489 (Table 3).
Group
N
Median
Interquartile Range
Min
Max
Z Statistic
P Value
SHED
9
26.66
27.57
6.60
37
-1.062
0.288
Control
9
16.21
25
4
37
Table 3: Comparing the percentage of blood vessel volume between SHED and control groups.
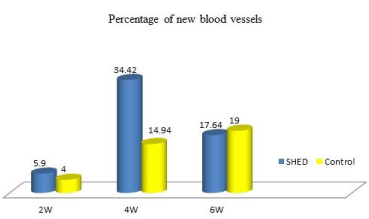
Figure 5: Percentage of new blood vessels in the central area of DO
regenerate (2W,2 weeks; 4W,4 weeks;6W,6 weeks).
Biochemical observation
Alkaline phosphatase levels were: Before operation ALP level for all rabbits were between 55 and 260 increased after 2 weeks to 86-290, the mean difference for SHED group was 128.33 and 42.66 for control group (Figure 6). The difference between the two groups was significant p< 0.001 (Table 4). Furthermore, we analyzed the correlation between the blood vessel parameters, bone volume data from the same biopsies and blood level of ALP. A positive correlation was found between the volume density of bone and blood vessels P= 0.014. Volume density of bone and ALP P= 0.029. No correlation between blood vessel volume and ALP level was observed, p=.372 (Table 5).
Group
N
Median
Interquartile range
Min
Max
Z Statistic
P Value
SHED
9
132
126.50
57
193
-3.536-
< 0.001
Control
9
41
17
31
57
Table 4: Comparing the percentage of serum ALP level between SHED and control groups.
Bone volume
Correlation Coefficient
Sig. (2-tailed)
Bone volume
Blood vessel volume
0.799
0.000
ALP level
BV volume
Correlation Coefficient
Sig. (2-tailed)
0.227
0.364
ALP level
Correlation Coefficient
Sig. (2-tailed)
0.520
0.027
Table 5: Correlation (Spearman) between bone volume, blood vessel volume and serum Alkaline phosphatase level.
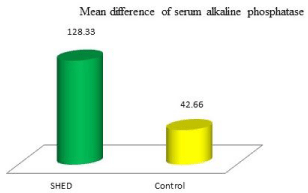
Figure 6: Mean difference of serum alkaline phosphatase level between
groups.
Discussion
In the present study we have chosen the central area of distracted mandible to compare a new bone formed and vascular area between the regenerate transplanted with SHED and the non transplanted control; because, this area has the least amount of bone formation [28]. The researchers proved that, in the central part of the distraction gap the callus remain fibrous four weeks after distraction, with collagen bundles oriented according to the direction of distraction, since bone formation and mineralization proceed with bone lamellae oriented along the predetermined network of collagen bundles growing from the margins of the osteotomy toward the center of the wound [29,30]. Recently, we have studied the influence of SHED transplanted in mandibular distraction osteogenesis in rabbit model for new bone formation. We have calculated the amount of new bone formed in the whole distracted area and compare it with non transplanted one [10]. The result demonstrated that more bone formation was observed in the distracted mandible transplanted with SHED than control. In biopsies, a very low amount of bone was formed in areas were characterized by a very scarce vascularization, given the relevance of blood vessels in the bone formation process. We hypothesized that the beneficial effect of a SHED on osteogenesis might have been caused by a better blood supply in the distraction gap which may also relate to SHED. In this study we examined more closely and quantified the area of newly formed bone and the blood vessels area present in the central region of the distraction gap of both groups. We hypothesized that 1) Addition of SHED have a stimulatory effect on new bone and blood vessel formation in the distraction gap and 2) Vascularization is positively correlated with a faster bone formation as seen in SHED group. The results revealed that SHED caused increase in the area of newly formed bone, and blood vessels, this may be described in terms of the stimulating effect of SHED on angiogenesis attributable to the increased number of vessels. The positive effect of SHED on vessels may be realized by a direct effect on the vessel endothelial cells or on the growth factors such as Vascular Endothelial Growth Factor (VEGF). The significant increase in the area of newly formed bone in SHED group is in agreement with several studies which suggest that, stem cells transplantation have a definite effect on promoting callus formation and shortening the consolidation period. Qi et al. [31] injected autologous bone marrow stem cells into distraction gaps of the rat mandible to accelerate callus formation, histological examination and histomorphometric analysis showed greater new bone formation and more mature bone following autologous cell transplantation. In orthopedic, similar results on transplantation of periosteum-derived osteoblast-like cells in the rabbit tibia lengthening model [32] and transplantation of marrow derived osteoblasts and progenitor cells in the rat femur distraction osteogenesis [33]. A clinical study also demonstrated that marrowderived mesenchymal stem cell transplantation greatly reduced the average healing index of the distraction gap from 38.7 to 23.0 days/cm in three cases with abnormities of the legs. Kitoh et al. [6] and Alkaisi et al. [10] transplanted SHED in mandibular DO, their results demonstrated a significant bone formation in the SHED group than control. Our results showed more vascularization in SHED group than control; however, it was statistically not significant. It has been found that Mesenchymal Stem Cells (MSCs) can secrete significant amounts of cytokines and growth factors [34,35], which promote new vessel formation by vessel dilators such as VEGF and nitric oxide and remodeling of injured tissues [36,37], to promote angiogenesis and tissue repairing. These observations led to the use of these cells for preclinical studies and clinical trials to treat ischemic limbs and hearts [38-41]. Recent studies have highlighted the ability of these cells to form capillary-like structures on their own [42], however Cyrus et al. [43] disagree with this result and they found in their in vitro study that, the culture conditions shown to induce HMSC differentiation to an endothelial-like phenotype do not match those of this study while Annabi et al. [42] believed, it is possible that HMSCs contributing to the enhanced network lengths observed by differentiating into endothelial cells and incorporating into the growing network. Huang et al. [44] found that stem cells promote growth and angiogenesis of tumors in mice. The secretion of interleukin-6 (IL-6) from MSCs demonstrate that the tumor microenvironment, namely, MSCssecreted IL-6, may enrich the proangiognic factors secreted by cancer cells to increase angiogenesis and tumor growth that targeting this interaction may lead to novel therapeutic and preventive strategies. Evidence implicates endothelial cell–mesenchymal cell crosstalk; endothelial cells have been shown to attract undifferentiated mesenchymal cells through paracrine mechanisms [45]. An important finding in this study is the positive correlation between the percentage of the amount of new bone formed and the volume density of the blood vessels , in agreement with Amir et al. [23], they found that in younger new bone area (area near the central part of DO) , there is positive correlation between the percentage of the amount of new bone formed and the volume density of the blood vessels. In distraction osteogenesis, bone is formed predominantly by intramembraneous ossification and osteogenesis takes place only in the area where blood vessels are present [46]. Cells that constitute the blood vessel walls or that are in the vicinity of the blood vessel wall are believed to secrete osteogenic factors and some of these cells appeared to have osteogenic potential [47-50]. Immunostaining with specific markers for vessel formation has shown that more precursor cells of new capillaries were present in the fibrous tissue area near locations where more bone was formed [46,49]. Poor regeneration of bone in the distraction zone may be due to problems related to either mobilization and expansion, or differentiation of primitive mesenchymal cells. This happens especially in patients who have a diminished pool of these primitive cells or in whom the host tissue bed has been compromised after severe trauma or radiotherapy [51]. Bone marrow is one of the possible sources [52], therefore, supplying osteogenic cells by means of autologous bone marrow stem cells seems to be a reasonable approach to guarantee bone regeneration which has several advantages, including being an easy procedure for volume expansion and osteogenic differentiation ex vivo, with an absence of toxic and immunoreactive side effects being autologous cells, and with a low risk of infection [6]. Our findings are in line with other studies on the role of blood vessels in the bone forming activity [46,49,53,54] and Amir et al. [22] provided an explanation as to why in a few of his patients with minimal vascularization of the transport bone segment, the new bone was very poorly developed. In the current study higher serum ALP level was found in SHED group than in control with significant positive correlation with new bone formation. As more has been learned about the biology of hard tissues and mineral metabolism, the role of ALP as a marker for osteogenic activity has been consistently solidified. In contrast, the detailed mechanism of how ALP functions in hard tissues is often considered controversial. ALP has many different functions in many organisms and tissues where it occurs, More than 80 years ago, the high level of ALP expression in bone was noted, and the first hypothesis put forward to explain why ALP is important for the hard tissue formation [55]. ALP is used as a marker of hard tissue cell differentiation or measurements of enzyme blood level [56]. Tissue engineering is an emerging area of interest in ALP, where, as in osteogenesis, success is measured by robust ALP expression, as it inevitably leads to mineralization of the neotissue [57-62]. Measurement of increased ALP expression enzymatic ally, histochemically or at the mRNA level is taken as a reliable indication of the osteoblastic, chondrocytic or odontoblastic phenotype, the function of this elevation in ALP expression is considered less clear. ALP is expressed early in development, and is soon observed on the cell surface and in matrix vesicles, later in the developmental program, while other genes (e.g. osteocalcin) are upregulated and ALP expression declines, clearly, ALP must function in the initial phases of the process. The mechanisms through which ALP expression is regulated are complex; a web of interlaced signaling pathways, the details of which are just emerging [56].
Conclusion
The transplantation of SHED during DO may contribute to improvements in wound healing by increasing osseous formation and vascularity with positive correlation between osteogenesis and angiogenesis as well ALP found to be an early biochemical marker to evaluate bone mineralization as its level increases during early DO, and its level have positive correlation with osteogenesis and angiogenesis. The results of this study suggest that this cell therapy accelerated callus and bone formation in mandibular osteodistraction, and therefore facilitated consolidation. It may be a valuable approach for clinical osteodistraction enhancement, especially in sites without enough osteogenic potential, e.g. for skeletal defect repair or in irradiated bone. However, we suggest that before clinical applications, new studies should determine the most effective number of SHED and application protocol for DO.
References
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003; 100: 5807-5812.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000; 97: 13625-13630.
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008; 34: 962-969.
- Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004; 15: 406-410.
- Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, et al. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006; 77: 1003-1007.
- Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone. 2004; 35: 892-898.
- Jiang X, Zou S, Ye B, Zhu S, Liu Y, Hu J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone. 2010; 46: 1156-1161.
- De Mendonca Costa, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, et al. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. Journal of Craniofacial Surgery. 2008; 19: 204-210.
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008; 14: 428-434.
- Alkaisi A, Abd Rashid Ismail, Samarendra S Mutum, Zainal A Rifin Ahmad, Sam'an Masudi, Noor Hayati AbdRazak. Transplantation of Human Dental Pulp Stem Cells: Enhance Bone Consolidation in Mandibular Distraction Osteogenesis. Journal of Oral and Maxillofacial Surgery. 2013; 71: 1758.e1–1758.e13.
- Carls FR, Jackson IT, Topf JS. Distraction osteogenesis for lengthening of the hard palate: Part I. A possible new treatment concept for velopharyngeal incompetence. Experimental study in dogs. Plast Reconstr Surg. 1997; 100: 1635-1647.
- Yu JC, Fearon J, Havlik RJ, Buchman SR, Polley JW. Distraction Osteogenesis of the Craniofacial Skeleton. Plast Reconstr Surg. 2004; 114: 1E-20E.
- Reddy LV, Elhadi HM. Maxillary advancement by distraction osteogenesis. Atlas Oral Maxillofac Surg Clin North Am. 2008; 16: 237-247.
- Nishimoto S, Oyama T, Nagashima T, Osaki Y, Yoshimura Y, Fukuda K, et al. Lateral orbital expansion and gradual fronto-orbital advancement: an option to treat severe syndromic craniosynostosis. J Craniofac Surg. 2008; 19: 1622-1627.
- Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1904. Clin Orthop Relat Res. 1994; 4-9.
- McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992; 89: 1-8.
- Karp NS, McCarthy JG, Schreiber JS, Sissons HA, Thorne CH. Membranous bone lengthening: a serial histological study. Ann Plast Surg. 1992; 29: 2-7.
- Ploder O, Kanz F, Randl U, Mayr W, Voracek M, Plenk H Jr. Three-dimensional histomorphometric analysis of distraction osteogenesis using an implanted device for mandibular lengthening in sheep. Plast Reconstr Surg. 2002; 110: 130-137.
- Zimmermann CE, Thurmüller P, Troulis MJ, Perrott DH, Rahn B, Kaban LB. Histology of the porcine mandibular distraction wound. Int J Oral Maxillofac Surg. 2005; 34: 411-419.
- Sencimen M, Aydintug YS, Ortakoglu K, Karslioglu Y, Gunhan O, Gunaydin Y. Histomorphometrical analysis of new bone obtained by distraction osteogenesis and osteogenesis by periosteal distraction in rabbits. Int J Oral Maxillofac Surg. 2007; 36: 235-242.
- Lawler ME, Tayebaty FT, Williams WB, Troulis MJ, Kaban LB. Histomorphometric analysis of the porcine mandibular distraction wound. J Oral Maxillofac Surg. 2010; 68: 1543-1554.
- Amir LR, Becking AG, Jovanovic A, Perdijk FB, Everts V, Bronckers AL. Formation of new bone during vertical distraction osteogenesis of the human mandible is related to the presence of blood vessels. Clin Oral Implants Res. 2006; 17: 410-416.
- Amir LR, Becking AG, Jovanovic A, Perdijk FBT, Everts V, Bronckers ALJ. Vertical distraction osteogenesis in the human mandible:a prospective morphometric study.Clinical Oral Implants Research. 2006; 17: 417-425.
- Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994; 15: 439-461.
- Moss DW. Physicochemical and pathophysiological factors in the release of membrane-bound alkaline phosphatase from cells. Clin Chim Acta. 1997; 257: 133-140.
- Goldstein DJ, Rogers CE, Harris H. Expression of alkaline phosphatase loci in mammalian tissues. Proc Natl Acad Sci USA. 1980; 77: 2857-2860.
- Antonella Liza Pantaleoni Andrietti. Compararive Osteogenesis of Equine Mesenchymal Stem Cells, Isolationfeom Bone Marrow, Adipose Tissue and Synocium. Master Thesis. VMS - Veterinary Clinical Medicine in the Graduate College of the University of Illinois at Urbana-Champaign. 2012.
- Dinu C, Kretschmer W, Baciut M, Rotaru H, Bolboaca SD, Gheban D, et al. The effect of distraction rate on bone histological and histomorphometrical properties in an ovine mandible model. Romanian Journal of Morphology and Embryology.2011; 52: 819-825.
- Karaharju EO, Aalto K, Kahri A, Lindberg LA, Kallio T, Karaharju-Suvanto T, et al. Distraction bone healing. Clinical orthopaedics and related research.1993; 297: 38-43.
- Cope JB, Samchukov ML. Regenerate bone formation and remodeling during mandibular osteodistraction. Angle Orthod. 2000; 70: 99-111.
- Qi M1, Hu J, Zou S, Zhou H, Han L. Mandibular distraction osteogenesis enhanced by bone marrow mesenchymal stem cells in rats. J Craniomaxillofac Surg. 2006; 34: 283-289.
- Tsubota S, Tsuchiya H, Shinokawa Y, Tomita K, Minato H. Transplantation of osteoblast-like cells to the distracted callus in rabbits. J Bone Joint Surg Br. 1999; 81: 125-129.
- Richards M, Huibregtse BA, Caplan AI, Goulet JA, Goldstein SA. Marrow-derived progenitor cell injections enhance new bone formation during distraction. J Orthop Res. 1999; 17: 900-908.
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004; 109: 1543-1549.
- Phinney DG, Hill K, Michelson C, DuTreil M, Hughes C, Humphries S, et al. Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells. 2006; 24: 186-198.
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109: 228-234.
- Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008; 295: H2308-2314.
- Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation.2005; 111: 150-156.
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006; 290: H2196-H2203.
- Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006; 24: 1620-1626.
- Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bonemarrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplan. 2009; 18: 371-380.
- Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003; 21: 337-347.
- Cyrus M Ghajar, Katherine S Blevins, Christopher CW Hughes, Steven C, George MD. Mesenchymal Stem Cells Enhance Angiogenesis in Mechanically Viable Prevascularized Tissues via Early Matrix Metalloproteinase UpregulationTssue Engineering. Mary Ann LiebertInc; 2006; 12.
- Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013; 32: 4343-4354.
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998; 141: 805-814.
- Li G, Simpson AH, Kenwright J, Triffitt JT. Effect of lengthening rate on angiogenesis during distraction osteogenesis. J Orthop Res. 1999; 17: 362-367.
- Reilly TM, Seldes R, Luchetti W, Brighton CT. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop Relat Res. 1998; 95-103.
- Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001; 153: 1133-1140.
- Lewinson D, Maor G, Rozen N, Rabinovich I, Stahl S, Rachmiel A. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem Cell Biol. 2001; 116: 381-388.
- Bronckers AL, Sasaguri K, Cavender AC, D’Souza RN, Engelse MA. Expression of Runx2/Cbfa1/Pebp2alpha a during angiogenesis in postnatal rodent and fetal human orofacial tissues. Journal of Bone and Mineral. Research. 2005; 20: 428-437.
- Holmes SB, Lloyd T, Coghlan KM, Newman L. Distraction osteogenesis of the mandible in the previously irradiated patient. J Oral Maxillofac Surg. 2002; 60: 305-309.
- Yoo JU, Johnstone B. Mesenchymal stem cells and musculoskeletal repair. CurrOpin Orthop. 2000; 11: 391-396.
- Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002; 17: 435-447.
- Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, et al. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004; 34: 849-861.
- Robison R, Soames KM. The Possible Significance of Hexosephosphoric Esters in Ossification: Part II. The Phosphoric Esterase of Ossifying Cartilage. Biochem J. 1924; 18: 740-754.
- Ellis E. Golub and Kathleen Boesze-Battaglia. The role of alkaline phosphatase in mineralization. CurrOpin Orthop.2007; 18: 444-448.
- El-Amin SF, Botchwey E, Tuli R, Kofron MD, Mesfin A, Sethuraman S, et al. Human osteoblast cells: isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J Biomed Mater Res A. 2006; 76: 439-449.
- Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006; 12: 1663-1673.
- George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng. 2006; 95: 404-411.
- Roostaeian J, Carlsen B, Simhaee D, Jarrahy R, Huang W, Ishida K, et al. Characterization of growth and osteogenic differentiation of rabbit bone marrow stromal cells. J Surg Res. 2006; 133: 76-83.
- Zhang X, Yang M, Lin L, Chen P, Ma KT, Zhou CY, et al. Runx2 over expression enhances osteoblastic differentiation and mineralization in adipose--derived stem cells in vitro and in vivo. Calcif Tissue Int. 2006; 79: 169-178.
- Chen B, Lin H, Zhao Y, Wang B, Zhao Y, Liu Y, et al. Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J Biomed Mater Res A. 2007; 80: 428-434.