
Research Article
Austin J Dent. 2016; 3(2): 1034.
Histochemical Analysis of Collagen Reorganization at the Tumor-Stromal Interface in Oral Squamous Cell Carcinoma- A Polarizing Microscopic Study
Alrani D, Niranjan KC*, Acharya S and Hallikeri K
Department of oral Pathology, SDM College of Dental Science and Hospital, India
*Corresponding author: Niranjan KC, Department of Oral Pathology, SDM College of Dental Science and Hospital, Dharwad, Karnataka, India
Received: March 23, 2016; Accepted: May 24, 2016; Published: May 26, 2016
Abstract
Stromal changes are the key factors for provision of nutrition and growth to any tumor and also they act as a barrier for spread of the tumor. There are limited histochemical studies in the literature on methods to detect, quantify and analyze the collagen in the invasive tumor front (ITF) of oral squamous cell carcinoma (OSCC).
Aim: 1) To analyze the nature of collagen (thickness, hue, density, birefringence) with respect to cohesive and discohesive tumor front of oral squamous cell carcinoma. 2) To correlate the nature of collagen at the different grades (Broder’s and Bryne’s grading system) of oral squamous cell carcinoma. 3) Clinicopathological correlation with the nature of collagen at the invasive front of oral squamous cell carcinoma.
Materials and Methods: Tissue sections of 30 OSCC cases with ITF were stained with hematoxylin and picrosirius red stains for evaluation of the nature of collagen under polarizing microscope.
Results: Tumor with cohesive front will have thick collagen fiber which is predominantly organized red- yellow in color, well packed and shows strong birefringence (p< 0.005). A gradual change in the nature of collagen fiber was observed in the discohesive tumor front where the collagen fiber were thin disorganized, yellow-orange to green-yellow in color loosely packed with weak birefringence (p< 0.005).
Conclusion: Cohesive tumor front with organized collagen fibers resist the tumor against invasion and metastasis, preventing it to increase in size and thus associated with initial stage of tumor (I&II) whereas in discohesive tumor front the fibers may enhance the movement of tumor cells towards invasion and metastasis.
Keywords: Collagen; Picrosirius Red (PSR); Oral Squamous Cell Carcinoma (OSCC); Invasive Tumor Front (ITF); Cohesive tumor front; Discohesive tumor front
Introduction
OSCC is composed of two discrete components, the malignant epithelial cells and the stroma in which they are dispersed [1]. One of the major aspects of tumor cell invasion and metastasis is the interaction between cancer cells and extracellular matrix component [2]. The stroma is composed of ground substance composed of proteoglycans, glycoproteins and water, the fibrous component including interstitial collagen and elastic fibers [3]. Extracellular matrix components are the key factors for nutrition and growth to any tumor and also they act as barrier for spread of the tumor [4]. Alteration in ECM may play a role in recurrence and in facilitation the invasion of tumor cells.
Collagen which constitutes 34% of the total extracellular matrix (ECM) proteins forms the integral part of connective tissue stroma and plays a vital role in maintaining structural integrity and in determining tissue function. The collagenous tissue in the stroma gives strength to the tumor by giving a skeleton to the tumor. Increase in collagen content of the extracellular matrix increases the mechanical stiffness and transport resistance of the tumors [3]. A recent study by Li, et al. in 2013 mentions that collagen fiber plays an important role in ECM destruction and remodeling [2], so the study of interstitial collagen has been the mainstay of investigative histological procedures in understanding the pathogenesis of the lesion.
Collagen has natural birefringence which is attributed to the arrangement of its fibers which is enhanced by special stains like van gieson, masson’s trichrome and picrosirius red. Picrosirius red stain is considered a highly specific and selective stain for collagen fibers due to its ability in differentiating between different types of collagen fibers in various pathological conditions.
There are limited histochemical studies in the literature on methods to detect, quantify and analyze the collagen in the tumor invasive front of oral squamous cell carcinoma (OSCC).
Aims and objectives
1. To analyze the nature of collagen (thickness, hue, density, birefringence) with respect to cohesive and discohesive tumor front of oral squamous cell carcinoma.
2. To correlate the nature of collagen at the different grades (Broder’s and Bryne’s grading system) of oral squamous cell carcinoma.
3. Clinicopathological correlation with the nature of collagen at the invasive front of oral squamous cell carcinoma.
Materials and Methods
Twenty nine histologically diagnosed cases of OSCC and two controls of normal mucosa were retrieved from the archives of Department of Oral Pathology, S.D.M College of Dental Sciences and Hospital, Dharwad. All the sections were subjected to haematoxylin and eosin and also picrosirius red (PSR) staining, Sirius Red F3B (C.I.35780). All the patients were subjected to radical surgery, accompanied by removal of lymph nodes conducted in S.D.M College of Dental Sciences and Hospital from year 2009 to 2015.
Majority of the OSCC cases showed discohesive tumor front 19 (63%) which is represented by high degree of tumor cell dissociation followed by cohesive tumor front 11 (37%) (Table 1), clinicopathological features of Oral Squamous cell carcinoma patients.
Parameter
Category
No of cases
Percentage
Gender
Male
24
83%
Female
5
17%
Age
<45
14
50%
>45
15
50%
Habit
T(tobacco)
18
63%
Smoking
5
17%
Combination
6
21%
Frequency of habit
0 year
5
17%
0-9 year
5
17%
10-19 year
9
30%
>20 year
10
36%
Size of tumor
< 4cm
17
59%
>4cm
12
41%
Site
BM,RMT,BGS
22
76%
T
6
20%
GIN,ALV
1
4%
Type
Exophytic
23
80%
Endophytic
6
20%
Stage
I + II
17
57%
III + IV
12
43%
Broder’s Grading
Well
20
67%
Mod, mod poor
9
33%
IFG grading
4-8
2
10%
9-12
11
37%
13-16
16
53%
Lymph node status
Negative (0)
20
70%
Positive (1)
9
30%
Pericapsular invasion
Negative (0)
21
73%
Positive (1)
8
27%
Surgical Margins
Negative (0)
25
87%
Positive (1)
4
13%
Pattern of invasion
Cohesive
10
37%
Discohesive
19
63%
Abbreviations: BM= Buccal Mucosa; RMT= Retromolar Trigone; BGS= Bucco-Gingival Sulcus; T= Tongue; ALV=Alveolus; GIN= Gingiva; Well= Well Differentiated Carcinoma; MOD= Moderately Differentiated Carcinoma; Mod-Poor= Moderately to Poorly Differentiated Carcinoma
Table 1: Categorical distribution of Clinicopathological features of Oral Squamous cell carcinoma patients.
Inclusion criteria: All the patients of OSCC who were subjected to radical surgery and accompanied by removal of lymph nodes.
Exclusion criteria: Incisional biopsies only, patients with lack of clinical details and areas of necrosis were not included in the study.
Analysis of collagen
All the PSR stained sections of OSCC were analyzed under Polarizing Microscope to observe and note the nature of collagen i.e. thickness, organization, hue, density, and birefringence irrespective the tumor is cohesive or discohesive at the ITF by 3 blind observer. The nature of collagen fibers was analyzed under the corresponding grades of carcinoma for both Broder’s and Bryne’s grading system. Data of each case was tabulated and were subjected for statistical analysis. Pearson’s correlation test was employed for comparison between various parameters in the different groups.
Results
In this study we found that there is positive correlation between the nature of collagen fiber in relation to patterns of invasive front. Tumor with cohesive front showed thick collagen fibers which are predominantly organized red- yellow in color, well packed and shows strong birefringence. A gradual change in the nature of collagen fiber was observed in the discohesive tumor front where the collagen fiber were thin disorganized, yellow-orange to green-yellow in color loosely packed with weak birefringence (p< 0.01) (Table 2).
Parameter
Categories
Cohesive
n=10
Discohesive
n=19
p-value
Thickness
Thick
11(100%)
4(21%)
0.000
Thin
0(0%)
15(79%)
Organization
Organized
10(91%)
0(0%)
0.000
Disorganized
1(9%)
19(100%)
Colors
Red –orange
11(100%)
3(16%)
0.000
Yellow-orange
0(0%)
3(16%)
Green-yellow
0(0%)
13(68%)
Density
Well packed
10(91%)
2(12%)
0.000
Loosely packed
1(9)
17(88%)
Birefringence
Strong
11(100%)
5(26%)
0.000
Weak
0(0%)
14(74%)
*=p-value is highly significant (< 0.01)
Table 2: Nature of collagen fiber in relation to patterns of invasive front.
Density of collagen fiber
When compared between the density of collagen fiber with the size of the tumor the result was statistically significant (p< 0.05). More that 50% of the lesions having size below 4 cm, collagen fiber were observed to be well packed and in more than 80% of lesions with size above 4 cm shows collagen fiber which were loosely packed (Table 3).
Density of collagen fiber was also seen to be statistical significant (p< 0.05) with TNM stage and metastasis of lymph node. The dense, well packed collagen fiber in the picrosirius stained section of more than 50% of cases of OSCC were noticed to be in stage I and II of TNM with absence of metastasis to lymph node whereas more than 80% of cases where collagen fiber were loosely packed were in stage III and IV of TNM with evident metastasis. Of all the parameter, density of packing has shown maximum correlation with Clinicopathological feature (Table 3).
Clinicopathological
Parameter
Parameter
Category
< 4cm
>4cm
p-value
SIZE OF TUMOR
Thickness
Thick
8(47%)
6(50%)
0.876
Thin
9(53%)
6(50%)
Organization
Organized
6(35%)
3(25%)
0.555
Disorganized
11(65%)
9(75%)
Colors
Red-orange
8(47%)
5(42%)
0.883
Orange-yellow
2(12%)
1(8%)
Yellow-green
7(41%)
6(50%)
Density
Well packed
9(53%)
2(17%)
0.047*
Loosely packed
8(47%)
10(83%)
Birefringence
Strong
10(59%)
5(42%)
0.362
Weak
7(41%)
7(58%)
TNM STAGE
Parameter
Category
I , II
III , IV
p-value
Thickness
Thick
9(53%)
6(46%)
0.713
Thin
8(47%)
6(54%)
Organization
Organized
7(41%)
3(23%)
0.297
Disorganized
10(59%)
9(77%)
Colors
Red-orange
8(47%)
6(46%)
0.921
Orange-yellow
2(12%)
1(8%)
Yellow-green
7(41%)
5(46%)
Density
Well packed
10(59%)
1(15%)
0.016*
Loosely packed
7(41%)
11(85%)
Birefringence
Strong
12(71%)
3(31%)
0.030*
Weak
5(29%)
9(69%)
METASTASIS
Parameter
Category
Absent
Present
p-value
Thickness
Thick
11(55%)
4(44%)
0.599
Thin
9(45%)
5(56%)
Organization
Organized
8(40%)
2(22%)
0.351
Disorganized
12(60%)
7(78%)
Colors
Red-orange
9(45%)
5(56%)
0.837
Orange-yellow
2(10%)
1(11%)
Yellow-green
9(45%)
3(44%)
Density
Well packed
11(55%)
1(11%)
0.026*
Loosely packed
9(45%)
8(89%)
Birefringence
Strong
11(55%)
4(44%)
0.599
Weak
9(45%)
5(55%)
*=p-value is significant (< 0.05)
Table 3: Clinicopathologiocal correlation with nature of collagen fibre.
Clinicopathological consideration
None of the other Clinicopathological parameter shows correlation with the advancing tumor front and nature of collagen fiber except age, size of tumor, TNM staging and metastasis of lymph node.
Histological grading system
Results were not statistically significant with Broder’s and Bryne’s grading system of OSCC but observation suggested that significant correlation was present. Majority of cases (>50%) which were graded as poor to moderate – differentiated according to Broder’s and Bryne’s grading system shows collagen fiber at the advancing tumor front as disorganized, loosely packed, greenish yellow in color with weak birefringence (Table 4).
Category
Parameter
Categories
Well
Mod, Mod-poor
p-value
Broder’s grading
Thickness
Thick
10(47%)
5(55%)
0.782
Thin
10(53%)
4(45%)
Organization
Organized
8(36%)
2(22%)
0.351
Disorganized
12(64%)
7(88%)
Colors
Red-orange
11(52%)
3(33%)
0.537
Yellow-orange
2(11%)
1(11%)
Green-yellow
7(37%)
5(56%)
Density
Well packed
9(42%)
3(33%)
0.657
Loosely packed
11(58%)
6(67%
Birefringence
Strong
11(58%)
3(33%)
0.225
Weak
8(42%)
6(67%)
Modified
Invasive front grading
Categories
4-8
9-12
13-16
Thickness
Thick
1(50%)
5(45%)
8(50%)
0.972
Thin
1(50%)
6(55%)
8(50%)
Organization
Organized
1(50%)
4(36%)
4(25%)
0.686
Disorganized
1(50%)
7(64%)
12(75%)
Colors
Red-orange
1(50%)
6(54%)
6(37%)
0.224
Yellow-orange
1(50%)
0
2(13%)
Green-yellow
0
5(46%)
8(50%)
Density
Well packed
1(50%)
4(36%)
6(37%)
0.934
Loosely packed
1(50%)
7(64%)
10(63%)
Birefringence
Strong
1(50%)
8(72%)
6(37%)
0.198
Weak
1(50%)
3(28%)
10(63%)
Abbreviations: Well= Well Differentiated Carcinoma; MOD= Moderately Differentiated Carcinoma; Mod-Poor= Moderately to Poorly Differentiated Carcinoma
Table 4: Correlation between the histological grading and nature of collagen fiber.
Discussion
One of the most prevalent keratinocyte based cancers is squamous cell carcinoma. The highly invasive and metastatic nature of OSCC makes it one of the most frustrating cancers to treat [5]. However, to be invasive, a tumor cell must be able penetrate and move through the stroma [6]. The process of tumor dissemination involves specific interactions with tumor cell-surface adhesion receptors and multiple adhesive components of the extracellular matrix (ECM).
Tumor stroma plays a critical role in carcinogenesis; to grow beyond a minimal size of 1-2 mm, the tumor requires stroma [4]. The stroma is composed of ground substance composed of proteoglycans, glycoproteins and water, and the fibrous component including interstitial collagen and elastic fibers [1]. Which provides the vascular supply, nourishment, oxygen supply, and also limits the influx of inflammatory cells, thereby acting as a barrier for immunological rejection. Fibrous component of stroma has shown to be associated with OSCC and hence stromal changes which indicate the propensity of tumor cells to infiltrate and metastasize are now being studied as one of the prognostic indicators [1].
Van Gieson and trichrome stains may not be ideal for detection of collagen fibers as both these methods fail to reveal thin collagen fibers, stain’s tendency to fade and these disadvantages lead to under estimation of collagen content. This perplexing issue incited Puchtler and colleagues to seek a better method and they found that Sirius red dissolved in a saturated picric acid solution (picrosirius red) consistently stained thin collagen fibers, did not fade, and was appropriate for use with polarized light microscopy [7].
Combination of sirius red and picric acid was first considered to be a special stain for connective tissue in 1964, especially for differentiating the subtype of collagen. It works on the principle that sulphonic group of sirius red reacts with the basic groups in collagen molecules. 126 sirius dye molecules bind to purified collagen types I, II and III. The enhanced birefringence of collagen is due to the attachment of the elongated dye molecules parallel to the long axis of the collagen. The orientation of the fibers to polars and collagen birefringence provides brightness to the collagen. Thickness, density of packing and spatial arrangement determines the polarization birefringence of the collagen. Picrosirius red stain thus helps in better understanding of the collagen function and pathology [8-11]. In addition the picrosirius red stained sections when observed under a polarizing microscope show birefringence due to the anisotropic properties of collagen [12,13].
As proposed in different studies that different patterns of invasion (representing different grades of tumor cell dissociation) are associated with the prognostic outcome in cancer [14]. An attempt has been made in this study to assess the nature of collagen at the invasive front of OSCC using picrosirius red and polarizing microscope.
Previous literature confirmed that Invasive front grading in contrast to Broder’s grading is of high prognostic value, because of the following reasons:
1. Tumors often are more poorly differentiated in invasive than in superficial parts [15].
2. Blood group H antigen is often lost in invasive tumor margins of OSCC and this lost is associated with poor prognosis [16].
3. Increased expression of proliferation associated structure in the invading tumor front [17].
4. Melanoma cells from the deep tumor parts have a higher DNA content than more superficial cells [18].
5. Increased expression of Ki-67, L-myc, c-myc, N,myc, c-erbB-2 oncoprotein in most invasive lung cancer [19,20].
6. Increased labeling of bromodeoxyuridine at the site of invasion [21].
All of these studies show that the key to a better understanding of the invasive and metastatic behavior of cancer cells may reside within the invasive margins of different tumors so in the present study nature of collagen fiber was analyzed with the modified invasive front grading.
As proposed by Bryne, et al. [22], pattern of invasion is divided into four grades,
Grade I: Pushing, well delineated infiltrating borders,
Grade II: Infiltrating, solid, cords, bands and/ or strands,
Grade III: Small groups or cords of infiltrating cells (n >15),
Grade IV: Marked and wide spread cellular dissociation in small groups and/ or in single cells (n< 15).
The cases were divided into 2 groups
a) The majority 19 (63%) of the tumor showed discohesive tumor front represented by grade II, III, IV (66%)
b) Followed by cohesive tumor front 11(37%) represented grade I.
In the present study with respect to the relationship between the collagenous component in the stroma and the invading tumor front, there have been some observable changes with different advancing tumor front i.e. cohesive and discohesive.
In cohesive tumor front densely packed distinct collagen, shows reddish orange color which was mainly concentrated around tumor islands probably to prevent the tumor invasion. This may be due to deposition of type 1 collagen fiber which were in the form of thick bands and composed of densely packed, well organized with strong birefringence (Figure 1A, 1B), while in discohesive tumor front collagen fiber were thin, loosely packed, disorganized, with weak birefringence, probably due to majority of Type III collagen fiber (Figure 2A, 2B, 3 and 4). This feature being consistent with the concept of Jungeria, et al. [23] and Montes, et al. [24]. They stated that the thick fibers were type I collagen fiber and exhibit an intense birefringence of red, orange and yellow color by polarizing microscope and a weak birefringence of green when fiber were thin fibrillar thus consisting type III collagen. Observation is further supported by study by Stenback, et al. [25], the presence of a delicate meshwork (reticular) of type III collagen at the invading front of tumor islands in increasing gradations of skin cancer. Study on “stromal reaction during tumor progression in oral mucosa” by Megumi Yokoyama revealed that type I collagen which was stained red by PSR, were decreased with advanced dysplastic grading [26].
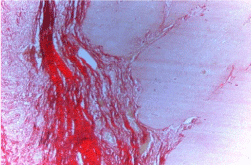
Figure 1A: Photomicrograph of Cohesive tumor front (Picro Sirius red stain
without polars, 10X).

Figure 1B: Photomicrograph of Cohesive tumor front (Picro Sirius red stain
with polars, 10X).
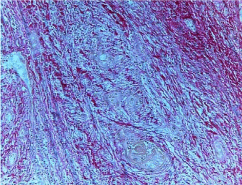
Figure 2A: Photomicrograph of Discohesive tumor front (Picro Sirius red
stain without polars, 10X).
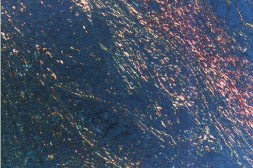
Figure 2B: Photomicrograph of Discohesive tumor front (Picro Sirius red
stain with polars, 10X).
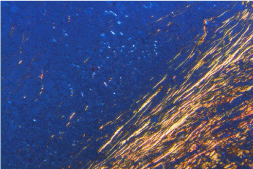
Figure 3: Photomicrograph of Discohesive tumor front showing thin collagen
fibers with yellow orange color birefringence (Picro Sirius red stain 10X).
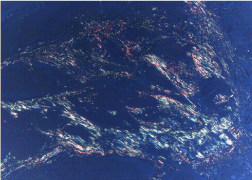
Figure 4: Photomicrograph of Discohesive tumor front showing thin,
disorganized, yellow orange color birefringence, loosely packed collagen
fibers. (Picro Sirius red stain 4X).
Thus the color changes observed in the present study clearly indicate some alteration in the stromal tissue around the tumor island of advancing front, which in turn may be due to carcinogenic agents that are involved in tumorigenesis. The above results are further supported by Brekken, et al. [27] who stated that the tumor progression is influenced by extracellular matrix. This finding is further supported by study on mechanics of capsule formation which reveals that a more robust extracellular matrix and capsule results in slower growth of tumor [28].
Further, nuclear resonance studies on the physical aggregation of the collagen fiber by Sharf, et al. [29] have also revealed a color profile of orange to red while corresponded to the well packed fiber and green to greenish yellow to poorly packed fibers. This collagen may be from the tumor cell in origin, thus benefiting the tumor by reducing the access to the host lymphocytes.
Alternatively, the collagen could be of stromal cell origin, thus benefiting the host by walling off the invading tumor.
Results in the study also revealed that 9 cases (53%), tumor having size less than 4 cm shows densely packed collagen fiber and 10 (83%) tumor having size greater than 4 cm shows loosely packed collagen fiber around the tumor island of advancing front. Hence tumors with excessive collagen in the stroma may respond in this manner as seen in study on breast cancer have also shown that an increase in collagen content of extracellular matrix increase the mechanical stiffness and transport resistance of tumor [30]. As also study on myocardial infarction concluded that the collagen degradation and loss after myocardial infarction is associated with infarct expansion and followed by functional decline [31].
Present study also revealed that majority of cases which were graded as TNM stage I and II 9 (56%) with absence of lymph node metastasis 10 (53%) shows densely packed collagen fiber whereas tumor in stage III and IV, 11 (85%) with evident metastasis 8 (89%), collagen fiber were loosely packed. Alon, et al. [32] in their study on stromal differences in salivary gland tumors found that 50% of collagen fiber in polymorphous low grade adenocarcinoma and adenoid cystic carcinoma were greenish yellow, whereas in pleomorphic adenoma, only 13% of them were greenish yellow. In similar manner study of SCC of skin and lower lip revealed that high grade of tumor cell dissociation, represented by a spray like pattern of invasion (PI), was significantly associated with a high frequency of metastatic as well as recurrent disease [33,34]. It was also reported that non-cohesive (spray like) pattern of invasion was significantly associated with the invasive with lymphovascular space involvement and large tumor size.
Contrary to our result the nature of collagen fiber was not significant with the Broder’s grading system as mentioned in the study done by Venigella [3] and Kalele [4], that collagen fiber in well differentiated carcinoma revealed polarizing colors of reddish orange around the tumor island, which gradually changes into yelloworange in moderately differentiated and greenish yellow with weak birefringence in poorly differentiated carcinoma, but association was observed with the invasive front grading by Bryne, et al. [35]. Previous literatures have also confirmed that Invasive front grading in contrast to the conventional Broder’s grading is of high prognostic value. Irrespective of difference scoring system the common findings of all study was that a discohesive tumor front was associated with metastasis, large tumor size, advanced tumor stage (TNM stage III and IV) increased recurrence and decrease survival.
Conclusion
Based on the above study it is concluded that picrosirius red stain with the use of polarizing microscope is most suitable stain to visualize collagen fibers and application of stain is a relatively simple tool to study the changes in extracellular matrix in particular the structural integrity of collagen fibers at the different invasive front of OSCC.
In the present study an observable change in the collagen with the different pattern of invasion was evinced with the pattern of invasion. Adjacent to the cohesive tumor front represented by pushing borders of invasion is positive correlated with thick bands of collagen which were well organized, and resist the tumor against invasion and metastasis, preventing it to increase in size and thus associated with initial stage of tumor (I&II). In discohesive tumor front the fibers are thin, disorganized which may enhance the movement of tumor cells towards invasion and metastasis.
Determination of collagen fiber nature in different patterns of invasion of oral squamous cell can help for targeting the stroma for various treatment strategies. Further research with larger sample size is with advanced techniques of using immunohistochemistry and collagen gene identification, Second harmonic generation (SHG) microscopy, Confocal laser microscopy are probably more specific and sensitive for collagen detection in this direction.
Drawbacks and Limitations
Though picrosirius red stains very thin collagen fibers in comparison to other collagen stains, Factors like pH, concentration of stain and the duration of staining will attribute to the variation in results.
Sample of the stains can be deteriorated when kept in for more than four years. Under this condition the solution loses its specificity and besides staining collagen, it also stains muscle and epithelium [11].
It is not advisable to use the staining technique on tissue preserved in formalin for many number of days. Hence, researchers must aim at ultra structural features of connective tissue in different stages of OSCC in future.
References
- Agrawal U, Rai H, Jain AK. Morphological and ultra structural characteristics of extracellular matrix changes in oral squamous cell carcinoma. Indian J Dent Res. 2011; 22:16-21.
- Manjunatha BS, Agrawal A, Shah V. Histopathological evaluation of collagen fibers using picrosirius red stain and polarizing microscopy in oral squamous cell carcinoma. J Can Res Ther. 2015; 11: 272-276.
- Aparna V, Charu S. Evaluation of collagen in different grades of oral squamous cell carcinoma by using the picrosirius red stain-A Histochemical Study. J Clin Diagn Res. 2010; 4: 3444-3449.
- Kalele KP, Managoli NA, Roopa NM, Kulkarni M, Bagul N, Kheur S. Assessment of collagen fiber nature, spatial distribution, hue and its correlation with invasion and metastasis in oral squamous cell carcinoma and surgical margins using Picro Sirius red and polarized microscope. J Dent Res Rev. 2014; 1: 14-17.
- Silverman S Jr. Oral cancer 4th ed. American Cancer Society. London B.C. Decker Inc.1998.
- Ziober BL, Turner MA, Palefsky JM, Banda MJ, Kramer RH. Type I collagen degradation by invasive oral squamous cell carcinoma. Oral Oncol. 2000; 36: 365-372.
- Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picrosirius red FBA. Beitr Pathol. 1973; 150: 174-187.
- Kamath VV, Satelur K, Komali Y, Krishnamurthy SS. Image analyses of collagen types and thickness in oral sub mucous fibrosis stained with picrosirius red under polarizing microscope. J Oro fac Sci. 2013; 5: 123-127.
- Coleman R. Picrosirius red staining revisited. Acta Histochem. 2011; 113: 231-233.
- Motes GS, Junqueira LC. The use of the Picrosirius – Polarization method for the study of the bio pathology of collagen. Mem Inst Oswald Cruz. 1991; 86: 1-11.
- Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochem J. 1989; 93: 27-29.
- Constantine VS, Mowry RW. Selective staining of human dermal collagen. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968; 50: 419-423.
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979; 11: 447-455.
- Horn LC, Fischer U, Raptis G, Hentschel B, Richter CE, Braumann UD, et al. Pattern of invasion is of prognostic value in surgically treated cervical cancer patients. Gynecol Oncol 2006; 103: 906-911.
- Hambraeus GM. Mercke CE, Willen R, Ranstam J, Samuelsson L, Lamm IL, et al. Prognostic factors influencing survival in combined radiation therapy and surgery of squamous cell carcinoma of the esophagus with special reference to a histopathologic grading system. Cancer.1988; 62: 895-904.
- Bryne M. Thrane PS. Dabelsteen E. Loss of expression of blood group antigen H is associated with cellular invasion and spread of oral squamous cell carcinomas. Cancer. 1991: 67: 613-618.
- Kearsely JH, Furlong KL, Waters MJ. An immune histochemical assessment of cellular proliferation markers in head and neck squamous cell cancers. Br J Cancer. 1990; 61: 821-827.
- LeBoit PE, Van Fletcher H. A comparative study of Spitz nevus and nodular malignant melanoma using image analysis cytometry. J Invest Dermatol. 1987; 88: 753 -757.
- Verhoeven D, Bourgeois N, Derde MP, Kaufman L, Buyssens N. Comparison of cell growth in different parts of breast cancers. Histopathology 1990; 17: 505-509.
- Roncalli M, Springall DR. Varndell IM. Oncoprotein immunoreactivity in human endocrine tumors. J Pathol.1991; 163: 117- 127.
- Kikuyama S. Kubota T. Watanabe M, Ishihiki K, Abe O. Cell kinetic study of human carcinomas using bromodeoxyuridine. Cell Tissue Kine. 1988; 21: 15-20.
- Bryne M, Koppang HS, Lilleng R, Stene T. Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinomas. J oral Pathol Med. 1989; 18: 432-437.
- Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by sirius red and polarization microscopy. Arch Histol Jpn. 1978; 41: 267-274.
- Montes GS, Krisztan RM, Shigihara KM, Tokoro R, Mourão PA, Junqueira LC. Histochemical and morphological characterization of reticular fibres. Histochemistry. 1980; 65: 131-141.
- Stenback F, Mäkinen MJ, Jussila T, Kauppila S, Risteli J, Talve L, et al. The extracellular matrix in skin tumor development-A morphological study. J Cutan Pathol. 1999; 26: 327-338.
- Megumi Yokoyama. Alterations in stromal reaction during tumor progression in oral mucosa. Journal of Hard Tissue Biology 2011; 20: 23-30.
- Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the extra cellular matrix. J Clin Invest. 2003; 111: 487-495.
- Lubkin SR, Jackson T. Multiphase mechanics of capsule formation in tumors. J Biomech Eng. 2002; 124: 237- 243.
- Sharf Y, Knubovets T, Dayan D, Hirshberg A, Akselrod S, Navon G. The source of the NMR detected motional anisotropy of water in blood vessel walls. Biophys J. 1997; 73: 1198-1204.
- Monsky WL, MoutaCarreira C, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenviourment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin can Res. 2002; 8: 1008-1013.
- Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation. 1991; 84: 2123-2134.
- Allon I, Vered M, Buchner A, Dayan D. Stromal differences in salivary gland tumors of a common histopathogenesis but with different biological behavior: a study with picrosirius red and polarizing microscope. Acta Histochem. 2006; 108: 259-264.
- Breuninger H, Schaumburg-Lever G, Holzschuh J, Horny HP. Desmoplastic squamous cell carcinoma of skin and vermilion surface: a highly malignant subtype of skin cancer. Cancer. 1997; 79: 915-919.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986; 20: 346-354.
- Bryne M, Koppang HS, Lilleng R, Kjerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J pathol. 1992; 166: 375-381.