
Research Article
Austin J Dent. 2017; 4(1): 1064.
Comparative Evaluation of Clinical Efficacy of Chlorhexidine, Phenolic and Chitosan Mouthrinses on Plaque and Gingivitis: Single-Centre, Double Blind, Randomized Controlled Clinical Study
Shah HP*, Bhavsar NV and Chavda MG
Department of Periodontia, Gujarat University, India
*Corresponding author: Shah HP, Department of Periodontia, Government dental college and hospital, Gujarat University, A/94, Natasha Park-2, Opp. Military boys hostel, Chhani road, Vadodara- 390002, Gujarat, India
Received: December 04, 2016; Accepted: January 20, 2017; Published: January 23, 2017
Abstract
The present study was conducted to determine the effect of different mouthwash preparations used as supplement to regular oral hygiene measures on plaque and gingivitis.
A total of 40 patients with chronic gingivitis demonstrating good oral hygiene were recruited for the study and divided into 4 groups. The subjects were briefed about the nature of the study and informed consents were taken. 4 different mouthwash preparations were given among the 4 groups. They are chlorhexidine digluconate, phenolic (Essential oil), chitosan anti-adhesive mouthwash and placebo (distilled water) for the control group. Clinical parameters including gingival index (Loe-Silness, 1963), plaque index (Turesky modification of Quigley-Hein, 1970) and stain index (Lobene, 1968) were recorded at baseline, 3 weeks and 6 weeks for all 4 groups.
Results showed that there is highly statistically significant decrease in plaque and gingival index in chlorhexidine and Essential oil group, statistically significant decrease in chitosan and placebo group. Findings from this study indicate chlorhexidine to be the most effective anti-plaque and anti-gingivitis agent, followed by Essential oil mouthwash with the benefit of reduced staining, thus can be used on long term basis, and whereas chitosan is not significantly effective compared to placebo group.
Keywords: Chlorhexidine; Phenolic; Chitosan; Mouthwash; Plaque; Gingivitis
Abbreviations
CHX: ChlorHeXidine; Ch: CHitosan; EO: Essential Oil
Introduction
The most common prevalent infectious oral diseases in humans are caries and periodontal diseases, including gingivitis and periodontitis, which are usually associated with dental plaque [1]. Removal of this bacterial biofilm is a decisive factor in the prevention as well as treatment of these diseases.
This specific form of biofilm can be negatively affected by plaque control. Plaque control includes the mechanical as well as chemical approaches to retard the plaque formation. Mechanical approaches include tooth brushing, interdental cleaning, using oral hygiene aids and professional prophylaxis. The effectiveness of this method mainly depends on the individual’s manual ability and motivation. However, the efficacy of mechanical debridement is limited by deep periodontal pockets, concavities, grooves and furcations in which pathogens persist. Moreover, recolonization of debrided sites by pathogenic bacteria from other intraoral niches has been reported [2]. This fact, couples with an increase in the information available on the microbiology of periodontal disease has stimulated a great interest in developing topical antimicrobial agents to control biofilm [3,4].
Over the years, a number of enzyme preparations, antiseptics (e.g., bisbiguanides, quaternary ammonium compounds, phenolic compounds, alkaloids, fluorides) and surface active agents have been developed and tested. [5,6].
Among the chemotherapeutic agents used in mouth-washes, chlorhexidine based formulations are currently the gold standard [7], with abundant evidence supporting its effectiveness. In spite of this proven effectiveness its long term use is limited because of some distinct adverse side effects like brownish staining of teeth, mucosal erosions [8].
Phenols and essential oils have been used in mouthrinses and lozenges for many years, which act via both a plaque inhibitory action and an anti-inflammatory action, possibly due to an anti-oxidative activity [9].
Chitosan, only natural polysaccharide that presents cationic character due to its amino groups which, at low pH, are protonated and can interact with negatively charged compounds such as proteins, anionic polysaccharides (e.g. alginates, carragenates, pectins), fatty acids, bile acids and phospholipids [10]. This behavior, along with its biocompatibility, biodegradability and lack of toxicity, has led to the usage of chitosan in diverse fields as technology, food, cosmetics, medicine, biotechnology, agriculture and the paper industry [11,12].
In recent years, a great attention has been devoted to the use of chitosan for pharmacological and biomedical applications. It exhibits various promising biological activities such as antimicrobial, antifungal, biodegradable, bio adhesive and biocompatible properties. It has shown excellent in vitro effect on bacterial biofilm as mouthrinse. So, naturally desirable next step would be testing in vivo clinical effectiveness. It is produced commercially by de acetylation of chitin, which is the structural element in the exoskeleton of crustaceans (crabs, shrimp, etc.) and cell walls of fungi [13].
Therefore, this study was undertaken with the purpose of exploring the potential of chitosan as a mouthrinse and comparing it with that of the established agents such as the ‘gold standard’ chlorhexidine and Essential oil mouthrinses on plaque and gingivitis.
Material and Methods
A group of 40 patients, 21 males and 19 females, in the age group of 16 to 50 years, from outpatient department at Department of Periodontia, Government dental college & hospital, Ahmedabad were recruited in the study.
Inclusion criteria
- Patients having chronic, inflammatory gingivitis,
- Presence of minimum of 20 natural teeth,
- No anterior teeth having restorations,
- Systemically healthy patients.
The patients with history of any systemic disorder, drug therapy, and parafunctional habits were excluded as they may alter the treatment plan or affect the outcome of the treatment. The patients with habits of tobacco chewing, smoking, or snuffing were excluded because of their established role in altering the soft tissue response, which may affect evaluation of clinical effect of mouthrinses on gingival health. Also, the stains produced by them, mask the true clinical effects of mouthwash on teeth staining.
Study protocol
At baseline, a complete intraoral soft tissue & hard tissue examination was performed and clinical parameters were recorded, including Plaque index (Turesky modification of Quigley & Hein index 1970) [14,15], gingival index (Loe & Silness 1963) [16] and Stain index (Modification of Lobene index 1968) [17] by the examiner in all the patients. They were rendered scaling and polishing along with root planing. They were instructed to continue to exercise their regular non-supervised, self-performed plaque control.
Patients were now divided into 4 groups. Group A (chlorhexidine), Group B (Essential oil), Group C (chitosan) and Group D (Placebocontrol). To avoid bias, this random division and allocation of respective mouthrinses were done by a junior resident student of the Periodontia department by withdrawing chit from box method. Resident was unaware of the study findings recorded by the examiner. Also, different mouthrinses were allocated in similar opaque white colored bottles for subject blinding. The members of control group and Essential oil group rinse vigorously with 20 ml for 30 s twice daily for 6 weeks. While chlorhexidine group and chitosan group rinse with 10 ml for 60 s twice daily for 6 weeks. At 3 weeks and 6 weeks after baseline, parameters were recorded again.
Materials used-Chlorhexidine mouthwash
Commercially available 0.2% chlorhexidine gluconate mouthwash. (Eludril perio, mouthwash, WIN Medicare pvt. Ltd, New Delhi, India)
Essential oil mouthwash
Commercially available Essential oil anticavity fluoride mouthwash (Listerine, Pfizer Consumer Healthcare, Morris Plains, New Jersey, USA)
Chitosan polymeric anti-adhesive mouthwash
Chitosan mouthwash formulation (Ch) was prepared using High Molecular Weight (DD >75%; MW 624 kDa) and Low Molecular Weight chitosan (75% < DD < 85%; MW 107 kDa) with the final concentration of either chitosan being 0.4% (v/v). The chitosan based mouthwash, prepared for a final pH of 5,contained 0.5% (w/v) salt (NaCl), 1% (w/v) stabilizer (arabic gum), 5% (w/v) sweetener (mannitol). Food grade flavoring and coloring were gently provided added at 0.1% (v/v) each.
(School of pharmacy, R.K University, Rajkot, Gujarat)
Placebo group
Distilled water.
Results and Statistical Analysis
The data obtained from the present study was suitably tabulated in appropriate tables. The mean and
Standard deviation was calculated for various parameters. The changes in clinical parameters from baseline, 3 weeks and 6 weeks were analysed among all the 4 groups using ANOVA test and adjusted p -values by performing bonferroni post-hoc test.
P-Value <0.05 is significant
P-Value <0.001 is highly significant
The result of the present study permitted following conclusions to be drawn:
There is highly statistically significant (p< 0.001) decrease in plaque index in chlorhexidine and essential oil mouthrinse groups (Table 1, Figure 1 and 2). There is highly statistically significant (p< 0.001) decrease in gingival index in chlorhexidine and essential oil mouthrinse groups (Table 2, Figure 1 and 2).
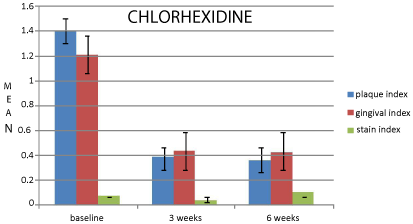
Figure 1: Comparison of mean changes in clinical parameters at baseline, 3
weeks and 6 weeks for chlorhexidine.
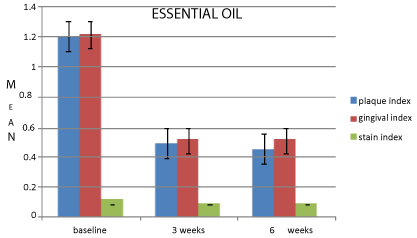
Figure 2: Comparison of mean changes in clinical parameters at baseline, 3
weeks and 6 weeks for essential oil.
As seen in previous meta-analysis [18], role of CHX on plaque and gingivitis is evident. Phenolic mouthrinses similarly have established role on plaque and gingival inflammation [19]. Recent clinical trials [20] conducted have shown effectiveness of EO mouthrinses as had been reported consistently in scientific reports.
There is statistically significant (p<0.05) decrease in plaque index in chitosan and placebo group (Table 1, Figure 3 and 4). There is statistically significant (p<0.05) decrease in gingival index in chitosan and placebo group (Table 2, Figure 3 and 4). Chitosan mouthrinse shows findings similar to that of placebo group, reflecting negligible in vivo clinical efficacy on plaque and gingivitis.
Group
N
Baseline
X ± SE
6 weeks
X ± SE
p value
1.4±0.1
chlorhexidine
10
0.36±0.1
<0.001
1.2±0.1
essential oil
10
0.45±0.1
<0.001
1.13±0.1
chitosan
10
0.9±0.1
<0.05
1.3±0.1
placebo
10
0.81±0.1
<0.05
(N= number of subjects, X ± SE= Mean ±standard error)
Table 1: Comparison of mean changes in plaque index at baseline and 6 weeks among chlorhexidine, essential oil, chitosan and placebo groups.
Group
N
Baseline
X ± SE
6 weeks
X ± SE
p value
Chlorhexidine
10
1.21±0.08
0.43±0.04
<0.001
essential oil
10
1.22±0.07
0.52±0.07
<0.001
Chitosan
10
1.19±0.08
0.77±0.08
<0.05
placebo
10
1.21±0.07
0.79±0.07
<0.05
(n= number of subjects, x ± se= mean ±standard error)
Table 2: Comparison of mean changes in gingival index at baseline and 6 weeks among chlorhexidine, essential oil, chitosan and placebo groups.
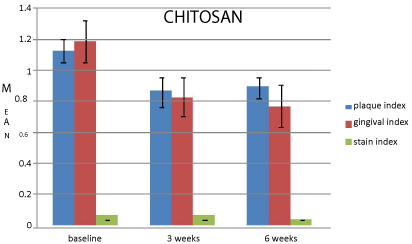
Figure 3: Comparison of mean changes in clinical parameters at baseline, 3
weeks and 6 weeks for chitosan.
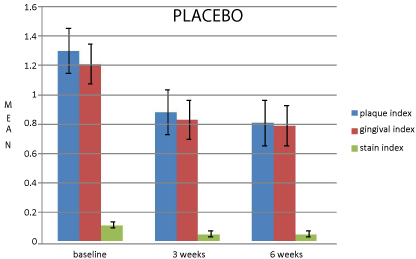
Figure 4: Comparison of mean changes in clinical parameters at baseline, 3
weeks and 6 weeks for placebo.
There is statistically significant (p<0.05) increase in stain index in chlorhexidine group. (Table 3, Figure 1). Data on stain index reflects tendency for brownish staining of chlorhexidine over a period of time, by proposed mechanisms shown by flora, et al. There is statistically insignificant (p>0.05) changes in stain index in essential oil, chitosan and placebo groups (Table 3, Figure 5, 6 and 7). Other mouthrinses have not shown staining property as a side-effect over a period of 6 weeks.
Group
N
Baseline
X ± SE
6 weeks
X ± SE
p value
Chlorhexidine
10
0.08±0.01
0.11±0.04
<0.05
essential oil
10
0.12±0.06
0.09±0.03
>0.05
Chitosan
10
0.07±0.02
0.04±0.02
>0.05
placebo
10
0.11±0.09
0.05±0.02
>0.05
Table 3: Comparison of mean changes in stain index at baseline and 6 weeks among chlorhexidine, essential oil, chitosan and placebo groups.
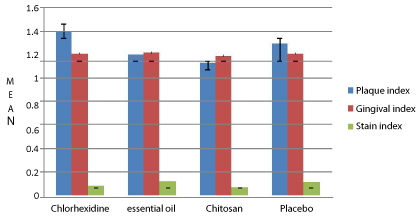
Figure 5: Results from the baseline examination with respect to plaque
(tures key modification of quigley & hein index), gingivitis(loe & silness;
gingival index) and extrinsic stain (lobene; stain index).
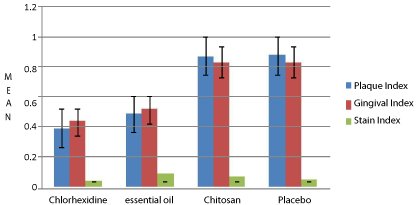
Figure 6: Results from 3 weeks examination with respect to plaque(tures
key modification of quigley & hein index), gingivitis (loe & silness; gingival
index) and extrins ic stain (lobene ; stain index).
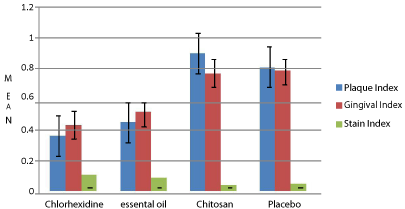
Figure 7: Results from 6 weeks examination with respect to plaque (tures
key modification of quigley & hein index), gingivitis (loe & silness; gingival
index) and extrinsic stain (lobene; stain index).
T?his controlled comparative clinical trial demonstrated that the essential oil mouthrinse and the chlorhexidine mouthrinse produced significant reductions in supragingival plaque and gingivitis when used as adjuncts to subjects’ usual mechanical oral hygiene procedures. However, chitosan mouthrinse and placebo control rinses showed negligible benefits in terms of these clinical parameters. Differences in clinical parameters between chitosan and placebo rinses are statistically insignificant.
Discussion
Most adults brush and floss inadequately, and constant education and/or reinforcement are often required [21]. Bacteria are usually left behind with mechanical oral health routines, and chemotherapeutic agents may have a key role as adjuncts to daily home-care.
To date, two antiseptic mouthwashes have received the ADA Seal of Acceptance: (CHX) chlorhexidine and Essential oil (Pfizer Consumer Healthcare, Morris Plains, New Jersey, USA; essential oil (EO) mouthwash).
CHX has a strong affinity for tooth and tissue surfaces, but can cause brown staining on the teeth and tongue. Patients must also wait until all traces of toothpaste are removed before rinsing with CHX.
Long term use of an EO mouthwash is microbiologically safe, with no changes observed in the bacterial composition of supragingival plaque, and no evidence of antimicrobial resistance. A number of trials have demonstrated the long-term plaque- and gingivitis -reducing properties of both CHX and EO mouthwashes [22].
Chitin is a linear polymer of N-acetyl glucosamine units linked by β-(1-4) bonds. It is the primary structural component of the shells of crustaceans, arthropods and fungal cell wall and is obtained mainly as a by-product of the fishing industry. It is considered one of the most abundant polysaccharides in nature after cellulose and its production is estimated at 109–1011 tons per year [23,24]. Partial deacetylation of chitin leads to chitosan, a polysaccharide composed of units of glucosamine (2-amino-2-deoxy-d-glucose) and N-acetyl glucosamine (2-acetamido-2-deoxy-d-glucose) linked by β (1?4) bonds.
Chitosan (CH), a natural polysaccharide obtained by the deacetylation of N-acetyl glucosamine, has received much more attention as a chemical agent for mouthwashes that provide clinical b enefits for plaque control. In addition to its favorable properties, such as nontoxicity, biocompatibility, and biodegradability, CH has an extended retention time on the oral mucosa [25]. Moreover; CH itself has an antimicrobial activity [26].
Recent studies have shown that chitosan has an in vitro antibacterial effect on Streptococcus mutans, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis [27]. It has also been reported that low-molecular-weight chitosan prevents the adsorption of S.mutans onto hydroxyapatite [28]. It is a widely distributed polycationic biopolymer, acts by interacting with the negatively charged bacterial cell membranes which leads to the leakage of proteinaceous and other intracellular constituents and alteration of cell permeability. At the present, no serious side effects of chitosan applications have been reported [26].
However, this study shows that in vitro effectiveness of chitosan doesn’t translate into clinical effect in vivo.
These findings add to the body of data supporting the effectiveness of chlorhexidine and essential oil mouthrinses [18-20]. While the two mouthrinses had comparable antigingivitis effectiveness, the chlorhexidine mouthrinse was statistically significantly more effective than the essential oil mouthrinse in reducing supragingival plaque. Results are consistent with the results of a previously conducted 6- month comparative study [29].
The occurrence of extrinsic stain and calculus deposition are recognized side effects of chlorhexidine mouthrinses [30] and may limit patient compliance with long -term use.
Therefore, it is likely that the chlorhexidine mouthrinse could have a greater role in situations when short-term plaque control is critical and usual mechanical oral hygiene procedures are difficult, e.g., in the immediate post-operative period after periodontal surgery, and the essential oil mouthrinse could have a role in the longer-term control of plaque and gingivitis during the maintenance phase of therapy.
In summary, the results of this investigation demonstrate that when used unsupervised as a part of regular oral hygiene and professional care, Chlorhexidine provides significantly greater plaque and gingivitis reductions than do rinses containing either phenolic compounds or chitosan. While essential oil rinses do provide modest plaque reduction benefits, their lack of extended anti gingivitis efficacy, when measured by well-established and objective scales, needs to be considered when choosing a treatment regimen.
Conclusion
In terms of all clinical parameters, chlorhexidine appears to be the best option, except for its staining side effect, which is maximum with it. Essential oil appears to be the second best option, regarding clinical parameters, along with less staining. Chitosan and control group produce similar results, and difference between them was statistically insignificant.
Findings from this study indicate chlorhexidine to be the most effective anti-plaque & anti gingivitis agent, but having very significant side effect of teeth staining after prolonged use which hampers it long term use clinically. Chlorhexidine is followed by Essential oil with the benefit of reduced teeth staining property, thus it can be used on long term basis , whereas chitosan is not significantly effective compared to placebo when used alone in vivo as anti-plaque & anti-gingivitis agent.
References
- Franco Neto CA, Parolo CC, Rosing CK, Maltz M. Comparative analysis of the effect of two chlorhexidine mouthrinses on plaque accumulation and gingival bleeding. Braz Oral Res. 2008; 22: 139-144.
- Decker EM, Von Ohle C, Weiger R, Wiech I, Brecx M. A synergistic chlorhexidine/chitosan combination for improved antiplaque strategies. J Periodontol Res. 2005; 40: 373-377.
- Ishikawa I, Baehni P. Nonsurgical periodontal therapy -- where do we stand now? Periodontol 2000. 2004; 36: 9-13.
- Umeda M, Takeuchi Y, Noguchi K, Huang Y, Koshy G, Ishikawa I. Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 2000. 2004; 36: 98-120.
- Quirynen M, Mongardini C, De Soete M, Pauwels M, Coucke W, Van Eldere J. The role of chlorhexidine in the one stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long -term clinical and microbiological observations. J Clin Periodontol. 2000; 27: 578-589.
- Pires JR, Rossa Junior C, Pizzolitto AC. In vitro antimicrobial efficiency of a mouthwash containing triclosan/gantrez and sodium bicarbonate. Braz oral Res. 2007; 21: 342-347.
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997; 15: 55-62.
- Greenstein G, Berman C, Jaffin R. Chlorhexidine. An adjunct to periodontal therapy. J Periodontol. 1986; 57: 370-377.
- Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004; 31: 878-884.
- Ramos VM, Rodriguez NM, Diaz MF, Rodriguez MS, Heras A, Agullo E. N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydrate polymers. 2003; 52: 39-46.
- Kim SK, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydrate Polymers. 2005; 62: 357-368.
- Kittur FS, Kumar AV, Gowda LR, Tharanathan RN. Chitosanolysis by a pectinase isozyme of Aspergillus niger-A non-specific activity. Carbohydrate polymers. 2003; 53: 191-196.
- No HK, Park NY, Lee SH, Meyers SP. Antimicrobial activity of chitosan and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002; 74: 65-72.
- Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970; 41: 41-43.
- Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962; 65: 26-29.
- Loe H. The gingival index, the plaque index and retention index systems. J Periodontol. 1967; 38: 610-616.
- Lobene RR. Effect of dentrifices on tooth stains with controlled brushing. J Am Dent Ass. 1968; 1: 77: 849-855.
- Herrera D. Chlorhexidine mouthwash reduces plaque and gingivitis. Evidence-based dentistry. 2013; 14: 17-18.
- Araujo MW, Charles CA, Weinstein RB, McGuire JA, Parikh-Das AM, Du Q, et al. Meta-analysis of the effect of an essential oil–containing mouthrinse on gingivitis and plaque. The Journal of the American Dental Association. 2015; 146: 610-622.
- Haas AN, Wagner TP, Muniz FW, Fiorini T, Cavagni J, Celeste RK. Essential oils -containing mouthwashes for gingivitis and plaque: Meta-analyses and meta-regression. J Dentistry. 2016; 55: 7-15.
- DePaola LG, Overholser CD, Meiller TF, Minah GE, Niehaus C. Chemotherapeutic inhibition of supragingival dental plaque and gingivitis development. J Clin Periodontol. 1989; 16: 311-315.
- Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol. 2003; 30: 13-16.
- Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Progress in polymer science. 2011; 36: 981-1014.
- Kurita K. Chemistry and application of chitin and chitosan. Polymer Degradation and stability. 1998; 59: 117-120.
- Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug -loaded chitosan microspheres. European Journal of Pharmaceutics and Biopharmaceutics. 2002; 53: 233-239.
- Sano H, Shibasaki Ki, Matsukubo t, Takaesu y. Effect of chitosan rinsing on reduction of dental plaque formation. The Bulletin of Tokyo Dental College. 2003; 44: 9-16.
- Boynueğri D, Özcan G, Senel S, Uç D, Uraz A, Öğüs E, et al. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: a pilot study. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009; 90: 461-466.
- Ikinci G, Senel S, Akincibay H, Kas S, Ercis S, Wilson CG, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002; 235: 121-127.
- Overholser CD, Meiller TF, DePaola LG, Minah GE, Niehaus C. Comparative effects of 2 mouthrinses on the development of supragingival dental plaque and gingivitis. J Clin Periodontol. 1990; 17: 575-579.
- Florta l, Gjermo p, Rolla g, Waerhaug j. Side effects of chlorhexidine mouthwas hes. Scand j dent res. 1972; 80:119-121.