
Review Article
Austin J Dent. 2021; 8(1): 1153.
Interdisciplinary Management of Bisphosphonate-Related Osteonecrosis of the Jaw
Szymacha K, Bielecka-Kowalska N* and Lewkowicz N
Department of Oral Mucosal and Periodontal Diseases, Medical University of Lodz, Poland
*Corresponding author: Natalia Bielecka-Kowalska, Department of Oral Mucosal and Periodontal Diseases Medical University of Lodz, Lodz, ul. Pomorska 251, 92- 217, Poland
Received: February 27, 2021; Accepted: March 18, 2021; Published: March 25, 2021
Abstract
Bisphosphonate (BP) Related Osteonecrosis of the Jaw (BRONJ) is one of the frequently occurring adverse reaction of BP intake, which is indicated in management of osteoporosis, Paget disease, multiple myeloma, and hypercalcemia in malignancies. The risk of osteonecrosis of the jaw relates to dose and duration of the therapy and the route of administration (i.e. more often BRONJ occurs in patients undergoing intravenous BPs therapy). At present, the management of BRONJ is a dilemma. No effective treatment has yet been developed. There is currently no gold standard of treatment for BRONJ. However, nowadays new alternative methods appear to be a promising modality of BRONJ treatment in early stages of the disease, while being safe and welltolerated, i.e. hyperbaric therapy, ozone therapy, use of platelet rich fibrine and platelet rich plasma, photodynamic therapy, low level laser therapy.
Keywords: Osteoporosis; Bisphosphonates; Osteonecrosis of the jaw; BRONJ; BPs; BPT
Abbreviations
BP: Bisohosphonate; BRONJ: Bisphosphonate Related Osteonecrosis of the Jaw; BMD: Bone Mineral Density; ALN: Alendronate; RIS: Risedronate; IBN: Ibandronate; SREs: Skeletal- Related Events; RANKL: Receptor Activator for Nuclear Factor κB Ligand; ONJ: Osteonecrosis of the Jaw; AAOMS: American Association of Oral and Maxillofacial Surgeons; MRONJ: Medication- Related Osteonecrosis of the Jaw; CTX: Plasma Terminal Telopeptide C; NTX: N-Terminal Telopeptide; PRF: Platelet Rich Fibrin; PRP: Platelet Rich Plasma; LLLT: Low-Level Laser Therapy
Introduction
Bisphosphonates (BPs) are currently widely used; however, recently recognized relationship of BPs with an array of pathologic conditions of skeletal disorders has brought increased scrutiny to the current broad use of BPs therapy. BPs are recommended primarily for diseases of osteoclast-mediated bone excessive bone loss due to osteoporosis, Paget disease of the bone, multiple myeloma, and hypercalcemia in malignancies. The therapy brings many positive clinical effects, but it is not without risk of significant adverse effects. Long standing clinical observations have revealed the association of this group of drugs with the osteonecrosis of the jaw [1].
Characteristics of Bisphosphonates
Bisphosphonates are potent inhibitors of osteoclast-mediated bone resorption, their mechanism of action is based on inhibiting osteoclast differentiation and blocking their activity. BPs have been shown to increase Bone Mineral Density (BMD), reduce bone turnover markers, and reduce the risk of osteoporotic fractures [2].
The BPs are divided due to their chemical structure and potency. The first-generation bisphosphonates (etidronate, clodronate) are characterized by short alkyl-side chains, and are no longer of clinical use. Second-generation BPs, such as alendronate and pamidronate, have amino-terminal groups and are known as aminobisphosphonates. Risedronate, a third-generation bisphosphonate, has a cyclic side chain. The antiresorptive properties increase approximately ten-fold between generations. Newer bisphosphonates, such as ibandronate, zoledronate, olpadronate and incandronate, are even more potent [3].
According to the American Society for Bone and Mineral Research, BPs are the most commonly used medications for prevention and treatment of postmenopausal osteoporosis in women and osteoporosis in men. They are also widely used in the treatment of inflammatory joint diseases and rheumatoid arthritis [4]. Between 2005 and 2009, approximately 150 million prescriptions were dispensed in the United States for the oral BPs Alendronate (ALN), Risedronate (RIS), or Ibandronate (IBN), and 5.1 million patients over the age of 55 years received a prescription for these drugs in 2008 [5].
The BPs are well tolerated in long-term treatment for over 3 years. In the patients with rheumatoid diseases, the preferred route of administration is intravenous injection [6-8]. Almost all (~94%) cases of BRONJ occur in patients receiving intravenous bisphosphonate as compared to oral bisphosphonates [9].
Bisphosphonates are widely utilized in cancer treatment by lowering the risk of bone loss associated with cancer therapy and the risk of disease recurrence, by managing hypercalcemia and by minimizing the risk of bone incidents in conditions with bone metastases.
The presence of such metastases is associated with increased skeletal morbidity due to Skeletal-Related Events (SREs), including radiation therapy to alleviate pain or prevent fracture, surgery to bone, pathological fracture and spinal cord compression, and also bone pain and hypercalcemia [10]. BPs are used in cases of breast cancer, prostate cancer, and multiple myeloma [11-13]. Less frequent indications are congenital osteogenesis imperfecta in children and Paget’s disease [2,5,9]. Bisphosphonate treatment does not completely effective in the treatment of SREs. Recently, a new antiresorptive drug was introduced on the market, denosumab. It is indicated in prevention of skeletal-related events in patients with bone metastases from solid tumors, for the treatment of postmenopausal osteoporosis and for treatment of cancer-induced bone loss in prostate or breast cancer patients, however, denosumab is not recommended for the prevention of SREs in patients with multiple myeloma [14]. Denosumab is a human monoclonal antibody that binds and inhibits the protein Receptor Activator for Nuclear factor κB Ligand (RANKL), an essential mediator in osteoclast formation, function and survival [10]. It exerts a strong antiresorptive effect, which is useful in cancer and osteoporosis patients in reducing skeletal related events. Due to the shorter half-life and lack of covalent bonding to the bone, it was expected that denosumab would have a similar therapeutic effect as BPs but with reduced profile of adverse effects, thus preventing cases of Osteonecrosis of the Jaw (ONJ). However, in 2010, several reports emerged describing the occurrence of ONJ in the patients being treated with denosumab [15-17].
The pathogenesis of BRONJ is not thoroughly understood and is undoubtedly multifactorial. Chronic pain and microtrauma of the jaws inhibit the healing process and as consequence prohibit bone resorption and its apposition. Some authors suggest that the microtraumas may not be fully recovered due to bisphosphonate inhibition of osteoclast-mediated bone resorption [2,9]. Osteoblasts develop brittle bones and the blood flow reduces due to the antiangiogenic effects of bisphosphonates, which all together contribute to necrosis or prevent bone healing.
In addition, bacteria-rich oral cavity environment facilitates infection, contributing to bone pain and inflammation.
Diagnosis of BRONJ
According to American Association of Oral and Maxillofacial Surgeons (AAOMS), the diagnosis of BRONJ is considered when all the following components are fulfilled [18,19]:
• Current or previous treatment with antiresorptive or antiangiogenic agents,
• Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than 8 weeks,
• No history of radiotherapy to the jaws or metastatic disease to the jaws (Figure 1).
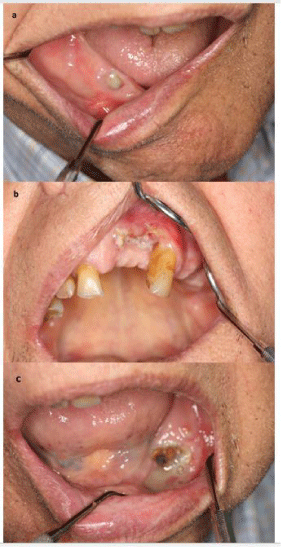
Figure 1: Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ)
following tooth extractions in the patient with multiple myeloma.
a: A necrotic bone exposure in the region of the tooth 44.
b: A necrotic bone exposure in the region of the teeth 11-22.
c: A necrotic bone exposure in the region of the teeth 33-35.
A revision of the nomenclature of bisphosphonate-related osteonecrosis of the jaw is recommended by AAOMS. The society favors the term Medication-Related Osteonecrosis of the Jaw (MRONJ). The change is justified to accommodate the growing number of osteonecrosis cases involving the maxilla and mandible associated with other antiresorptive (denosumab) and antiangiogenic therapies [18,19].
American Association of Oral and Maxillofacial Surgeons classified BRON into three stages and offered guidelines in the diagnosis and treatment of BRON (Table 1) [18,19]. It is estimated that BRONJ occurs in roughly 20% of patients receiving intravenous zoledronic acid for cancer therapy and in between 0-0.04% of patients taking orally administered bisphosphonates [18,19].
Classification
Treatment
Stage 1
Exposed bone, but no pain or sign of infection is visible.
No surgical treatment is required.
Oral antimicrobial rinses, such as chlorhexidine 0.12% is indicated.
Pain control may also be advisable.
Patients should be followed up every 3-4 months.Stage 2
Symptoms of pain are reported, or signs of infection.
Pain control may also be indicated.
The use of oral antimicrobial rinses in combination with oral systemic antibiotic therapy (penicillin, metronidazole, quinolones, clindamycin, doxycycline and erythromycin) is advised.Stage 3
Presence of pain or infection; fracture, fistula, or osteolysis can also be noted.
The therapy requires surgical debridement of necrotic bone, antimicrobial therapy (oral or intravenous), and analgesia and routine oral antimicrobial rinses (0.12% chlorhexidine).
Table 1: Classification and treatment of BRONJ according to the American Association of Oral and Maxillofacial Surgeons.
Prevention
Routine antibiotic prophylaxis is recommended in patients treated with bisphosphonates, especially if procedures involve tooth extraction, endodontic or periodontal therapies of the alveolar process. The Polish Dental Association and The National Antibiotic Awareness Program for using antibiotics in dentistry, advice usage of the antibiotics as follows: the first dose should be taken one the day before the procedure and then the course should continue for three consecutive days (so-called short-term prophylaxis). This recommendation suggests to prescribe amoxicillin with clavulanic acid: (at a dose 1000mg (875mg + 125mg) every 12 hours. In patients with allergy to penicillin, clindamycin (at a dose of 300mg every 8 hours) should be administered instead. As dental treatment may result in osteonecrosis incidents, the procedures should be as atraumatic as possible. It has been proven that the primary closure of the extraction wound has a large impact on the possible occurrence of osteonecrosis, which is why more and more attention is paid to methods that may affect the blood supply of the alveolar bone to accelerate healing [20]. The AAOMS has issued the following recommendations for patients taking oral bisphosphonates (Table 2) [21]. Similar prevention strategies appear to be appropriate in patients receiving other antiresorptive therapies.
Patients with no clinical risk factors on BPs treatment for less than 3 years
Patients exposed to less than 3 years of BPs with concomitant corticosteroids
Patients exposed to BPs for more than 3 years without any steroid or prednisone use
No alteration or delay in the planned surgery is necessary consent for dental implant surgery should be obtained relating to possible future implant failure and possible ONJ regular recall schedule.
Physician should be contacted discontinuation of the oral bisphosphonate for 3 months before surgery bisphosphonates may be resumed after osseous healing.
Physician should be contacted discontinuation of the oral bisphosphonate for 3 months before surgery bisphosphonates may be resumed after osseous healing.
Table 2: Recommendations for patients taking oral bisphosphonates issued by AAOMS.
Although the dental implant surgery-related BRONJ is rare, the possibility of occurrence is present. Lazarovici et al. described that out of 145 patients diagnosed with BRONJ, 27 patients received dental implants during BP therapy; 11 (41%) patients developed BRONJ being on oral BPs and 16 (59%) developed BRONJ associated with intravenous BPs [22]. The same authors suggest that the development of BRONJ associated with dental implants is a late complication, thus, patients treated with BPs who receive dental implants should be followed for a long period.
In the patients receiving BPs, special prophylactic measures to avoid extraction should be taken, i.e. routine dental check-ups, regular scaling sessions, additional use of topical fluoride and lowcarbohydrate diet to prevent caries. In the patients treated with oral BPs for less than 3 years with a coexisting minimum one risk factor (i.e. including those with greater cumulative bisphosphonate exposure (>4 years), and those with comorbid risk factors such as rheumatoid arthritis, prior or current glucocorticoid exposure, diabetes and smoking until the site has healed [18], it is advisable to test plasma Terminal Telopeptide C (CTX) level [23]. The CTX level could help to determine the BRONJ risk [24]. Low risk of osteonecrosis occurs at CTX above 150pg/ml, whereas the risk is high when the level of CTX is below 100pg/ml. However, available literature provide conflicting information about diagnostic effectiveness of CTX marker [23,25,26].
Temporary suspension of BPs does not provide a short-term advantage, whereas long-term suspension (if systemic conditions allow it) may be effective in stabilizing BRONJ sites and the clinical symptoms [19]. When BP therapy is discontinued, it was suggested to check bone turnover markers CTX (C-Terminal Telopeptide), NTX (N-Terminal Telopeptide), parathyroid hormone, and 1,25-dihydroxy vitamin D before surgical procedures [23-25]. The antiresorptive effect of denosumab can be dissipated within 6 months of discontinuation of the medication. There are, however, no studies to support or refute the strategy of discontinuation of denosumab therapy in MRONJ prevention or treatment [18].
Treatment
As a part of the treatment, antibacterial rinses, e.g. 0.12-0.2 % chlorhexidine, should be included in addition to the systemic antibiotics. Superficial removal of necrotic bone or hyperbaric oxygen therapy is sometimes beneficial [27]. In Stage I: No surgical treatment is indicated. Patients benefit from oral antimicrobial rinses, such as chlorhexidine 0.12%, and do well with this type of conservative treatment. Patients should be followed up every 3-4 months [28].
The use of oral antimicrobial rinses in combination with oral systemic antibiotic therapy (penicillin, metronidazole, quinolones, clindamycin, doxycycline, and erythromycin) is indicated for Stages II and III of AAOMS Staging. Most of the isolated bacteria species have been sensitive to the penicillins. For those with a penicillin allergy, quinolones, metronidazole, clindamycin, doxycycline, and erythromycin can be dispensed. Microbial cultures should also be analyzed for the presence of Actinomyces spp. If the Actinomyces microbe is isolated, then the antibiotic regimen can be adjusted. In some refractory cases, patients may require combination antibiotic therapy, long-term antibiotic maintenance, or a course of intravenous antibiotic therapy. Pain control may also be indicated. In Stage III the therapy requires surgical debridement of necrotic bone, antimicrobial therapy (oral or intravenous), analgesia and routine oral antimicrobial rinses (0.12% chlorhexidine) (Figure 2).
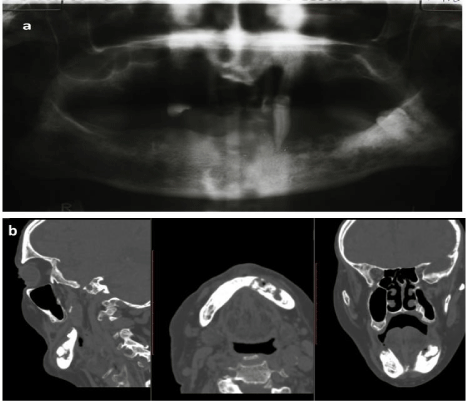
Figure 2: Typical images of bone sequestration and periosteal reaction in the
patient with BRONJ Grade 3.
a: Orthopanoramic radiograph.
b: Fan-beam computed tomography scans.
Regardless of the stage of the disease, mobile segments of bony sequestrum should be removed without exposing the uninvolved bone. The extraction of symptomatic teeth within exposed, necrotic bone should be considered because it is unlikely that the extraction will worsen the necrotic process.
Surgical treatment, in accordance to the AAOMS Position Paper, is reserved to patients affected by Stage III of BRONJ even if in the last version (2009) a superficial debridement is indicated to relieve soft tissue irritation also in the stage II (lesions being unresponsive to antibiotic treatment) [21]. Aggressive surgical treatment may occasionally results in even larger areas of exposed and painful infected bone. Surgical debridement or resection in combination with antibiotic therapy may offer long-term palliation with resolution of acute infection and pain. Mobile segments of bony sequestrum should be removed without exposing unaffected bone. If pathological fractures or complete mandibular involvement are observed, the affected bone portion may be respected and primary bone reconstruction or revascularization graft may be carried out [18,19,28].
Patients who develop BRONJ associated with dental implants should undergo long-term treatment with doxycycline 100 to 200 mg/d, and their dental implants should be removed only if the antibiotic treatment fails to alleviate the signs and symptoms of BRONJ [22].
The role of hyperbaric oxygen therapy is still unclear but some benefits of this treatment have recently been described in association with discontinuation of BPs and conventional therapy (medical or/ and surgical) [27]. The new regimens for hyperbaric therapy are still being sought. In the study in rats, the hyperbaric oxygen used immediately after tooth extraction resulted in reduced development of bone necrosis associated with ingestion of zoledronate [29]. Ozone therapy is another option in the management of bone necrosis or in extraction sites during and after oral surgery in patients treated with BPs. Ozone application was demonstrated to stimulate cell proliferation and soft tissue healing [6].
A new alternative treatment of osteonecrosis after tooth extraction is the use of adipose stem cells to graft the alveolus. In the mouse model, a great bone regenerative potential has been demonstrated [8]. The research on rabbits also confirmed the regenerative potential of adipose derived stem cells, primarily by accelerating the healing of gingival epithelium [30]. Another method of treatment enhancing bone healing is the use of growth factors in the form of Platelet Rich Fibrin (PRF) and/or Platelet Rich Plasma (PRP). Several studies showed the advantages of PRF application compared to PRP [7,31,32].
An in vitro study suggested that calcium phosphate had a protective effect on the cytotoxicity of zoledronic acid. It may be used locally for surgical wound care and may potentially reduce the risk of BRONJ in patients undergoing bisphosphonate therapy [33].
Due to its antimicrobial and anti-inflammatory effects, Photobiomodulation Therapy (PBMT) is another alternative method used in BRONJ care. Low-Level Laser Therapy (LLLT) will been employed as an adjunctive therapy. Photobiomodulation effect of LLLT involves an increase in inorganic matrix of bone and osteoblast mitotic index, and stimulation of lymphatic and blood capillary growth [34]. Some studies revealed a beneficial outcome of combining LLLT and PRF in the treatment of BLONJ [19,35,36]. Er:YAG laser may be used for minimally invasive surgery, vaporizing the necrotic bone before healthy bone is reached. In addition, Er:YAG laser possesses bactericidal and photobiomodulatory effect, accelerating soft and bone tissue healing in comparison to conventional treatments [35].
Some studies have demonstrated that vitamin D deficiency has been linked with the incidence of osteoporosis [37,38]. In addition, vitamin D deficiency has been reported as responsible for 18-35 per cent of bisphosphonate-treated cases, which have resulted in ineffective treatment of osteoporosis [39]. A number of authors have indicated that adequate levels of vitamin D can decrease bone resorption, counteract increased levels of parathyroid hormones, and have a positive effect on neuromuscular performance and risk of falling and bone strength. Therefore promotes the use of vitamin D supplies in conjunction with bisphosphonate therapy for prophylactic or medicinal reasons [37,40].
The use of bisphosphonate and the combination of bisphosphonate + vitamin D have been shown to histologically enhance the healing of fractures compared to vitamin D supplementation alone and in the control group. Consequently, it has been found that the initiation of bisphosphonate therapy following injury has a favorable impact on fracture healing. Without adverse effects on fracture repair, bisphosphonate treatment combined with vitamin D can also easily be used [37].
In conclusion physicians should continue to prescribe adequate supplies of vitamin D within the treatment target; for example, more than 50nmol/l during bisphosphate therapy [40].
Conclusions
Since ONJ may occur in patients receiving BPs and denosumab, dental consultation is advisable before introducing pharmacological therapy. Moreover, patients should avoid invasive dental procedures during treatment with antiresorptive drugs. At present, the management of BRONJ is a dilemma. No effective treatment has yet been developed, and it does not seem beneficial to discontinue treatment with BPs. Long-term, prospective studies are required to establish the efficacy of drug holidays in reducing the risk of BRONJ for patients receiving oral BPs. However, it must be recognized that interindividual variability, gender, age, physical activity, and seasonal and circadian variation exist that can result in difficulty in interpreting the results and more research is needed. A multidisciplinary approach to BRONJ prevention is recommended in the treatment of patients needing bisphosphonate therapy, with both patient and health professional training on the risks of developing BRONJ. The education of dentists, pharmacists, medical practitioners and patients on BRONJ should put strong emphasis on providing more preventative measures and advice on oral hygiene.
References
- Body JJ, Bartl R, Burckhardt P, Delmas PD, Diel IJ, Fleisch H, et al. Current use of bisphosphonates in oncology. International Bone and Cancer Study Group. J Clin Oncol. 1998; 16: 3890-3899.
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc. 2008; 83: 1032-1045.
- Sietsema WK, Ebetino FH, Salvagno AM, Bevan JA. Antiresorptive doseresponse relationships across three generations of bisphosphonates. Drugs Exp Clin Res. 1989; 15: 389-396.
- Hodgson SF, Watts NB, Bilezikian JP, Clarke BL, Gray TK, Harris DW, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003; 9: 544-564.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016; 31: 16-35.
- Agrillo A, Ungari C, Filiaci F, Priore P, Iannetti G. Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. J Craniofac Surg. 2007; 18: 1071-1075.
- Al-Hamed FS, Mahri M, Al-Waeli H, Torres J, Badran Z, Tamimi F. Regenerative Effect of Platelet Concentrates in Oral and Craniofacial Regeneration. Front Cardiovasc Med. 2019; 6: 126.
- Alonso-Rodriguez E, González-Martín-Moro J, Cebrián-Carretero JL, Del Castillo JL, Pozo-Kreilinger JJ, Ruiz-Bravo E, et al. Bisphosphonate-related osteonecrosis. Application of adipose-derived stem cells in an experimental murine model. Med Oral Patol Oral y Cir Bucal. 2019; 24: e529-e536.
- King AE, Umland EM. Osteonecrosis of the jaw in patients receiving intravenous or oral bisphosphonates. Pharmacotherapy. 2008; 28: 667-677.
- Scott LJ, Muir VJ. Denosumab: in the prevention of skeletal-related events in patients with bone metastases from solid tumours. Drugs. 2011; 71: 1059- 1069.
- Nussbaum SR, Younger J, Vandepol CJ, Gagel RF, Zubler MA, Chapman R, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: Comparison of 30, 60 and 90 mg dosages. Am J Med. 1993; 95: 297-304.
- Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001; 19: 558-567.
- Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of Pamidronate in Reducing Skeletal Complications in Patients with Breast Cancer and Lytic Bone Metastases. N Engl J Med. 1996; 335: 1785- 1792.
- Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body JJ, et al. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res. 2008; 14: 6387-6395.
- Qaisi M, Hargett J, Loeb M, Brown J, Caloss R. Denosumab Related Osteonecrosis of the Jaw with Spontaneous Necrosis of the Soft Palate: Report of a Life Threatening Case. Case Rep Dent. 2016; 2016: 5070187.
- Taylor KH, Middlefell LS, Mizen KD. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg. 2010; 48: 221-223.
- Pichardo SEC, Kuypers SCC, van Merkesteyn JPR. Denosumab osteonecrosis of the mandible: a new entity? A case report. J cranio-maxillofacial Surg. 2013; 41: e65-e69.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J oral Maxillofac Surg. 2009; 67: 2-12.
- Vescovi P, Nammour S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A critical review. Minerva Stomatol. 2010; 59: 181-203, 204-213.
- Kaczmarzyk T, Babiuch K, Dominiak M, Konopka T, Lipski M, Olczakkowalczyk D, et al. Rekomendacje Grupy Roboczej Polskiego Towarzystwa Stomatologicznego i Narodowego Programu Ochrony Antybiotyków w zakresie stosowania antybiotyków w stomatologii. WwwAntybiotykiEduPl. 2019.
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J oral Maxillofac Surg. 2014; 72: 1938-1956.
- Lazarovici TS, Yahalom R, Taicher S, Schwartz-Arad D, Peleg O, Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J oral Maxillofac Surg. 2010; 68: 790-796.
- Marx RE, Cillo JEJ, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J oral Maxillofac Surg. 2007; 65: 2397-2410.
- Dal Prá KJ, Lemos CAA, Okamoto R, Soubhia AMP, Pellizzer EP. Efficacy of the C-terminal telopeptide test in predicting the development of bisphosphonate-related osteonecrosis of the jaw: a systematic review. Int J Oral Maxillofac Surg. 2017; 46: 151-156.
- Fleisher KE, Welch G, Kottal S, Craig RG, Saxena D, Glickman RS. Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110: 509-516.
- Awad ME, Sun C, Jernigan J, Elsalanty M. Serum C-terminal cross-linking telopeptide level as a predictive biomarker of osteonecrosis after dentoalveolar surgery in patients receiving bisphosphonate therapy: Systematic review and meta-analysis. J Am Dent Assoc. 2019; 150: 664-675.e8.
- Freiberger JJ, Padilla-Burgos R, McGraw T, Suliman HB, Kraft KH, Stolp BW, et al. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics? J oral Maxillofac Surg. 2012; 70: 1573-1583.
- Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res. 2007; 86: 1013-1021.
- Liu SS, Lin TY, Fu E, Hsia YJ, Chiu HC, Tu HP, et al. Immediate hyperbaric oxygen after tooth extraction ameliorates bisphosphonate-related osteonecrotic lesion in rats. J Periodontol. 2019; 90: 1449-1456.
- Zang X, He L, Zhao L, He Y, Xiao E, Zhang Y. Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor β-1-mediated gingival wound healing. Stem Cell Res Ther. 2019; 10: 169.
- Steller D, Herbst N, Pries R, Juhl D, Hakim SG. Positive impact of Plateletrich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci Rep. 2019; 9: 8310.
- Soydan SS, Uckan S. Management of bisphosphonate-related osteonecrosis of the jaw with a platelet-rich fibrin membrane: technical report. J oral Maxillofac Surg. 2014; 72: 322-326.
- Paulo S, Laranjo M, Abrantes AM, Casalta-Lopes J, Santos K, Gonçalves AC, et al. Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Mater (Basel, Switzerland). 2019; 12: 1840.
- Minamisako MC, Ribeiro GH, Lisboa ML, Mariela Rodríguez Cordeiro M, Grando LJ. Medication-Related Osteonecrosis of Jaws: A Low-Level Laser Therapy and Antimicrobial Photodynamic Therapy Case Approach. Case Rep Dent. 2016; 2016: 6267406.
- Mauceri R, Panzarella V, Maniscalco L, Bedogni A, Licata ME, Albanese A, et al. Conservative Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Er, Cr: YSGG Laser and Platelet-Rich Plasma: A Longitudinal Study. Biomed Res Int. 2018; 2018: 3982540.
- Stübinger S. Advances in bone surgery: the Er: YAG laser in oral surgery and implant dentistry. Clin Cosmet Investig Dent. 2010; 2: 47-62.
- Aydogan NH, Ozel I, Iltar S, Kara T, Ozmeriç A, Alemdaroglu KB. The effect of vitamin D and bisphosphonate on fracture healing: An experimental study. J Clin Orthop trauma. 2016; 7: 90-94.
- Mezquita-Raya P, Muñoz-Torres M, Luna JD, Luna V, Lopez-Rodriguez F, Torres-Vela E, et al. Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J bone Miner Res. 2001; 16: 1408-1415.
- Díez-Pérez A, Gonzalez-Macías J. Inadequate responders to osteoporosis treatment: proposal for an operational definition. Osteoporos Int. 2008; 19: 1511-1516.
- Lems WF, Geusens P. Are bisphosphonates effective and safe in patients with low serum vitamin D levels? Int J Clin Rheumtol. 2009; 4: 119-121.