
Research Article
Ann Depress Anxiety. 2014;1(3): 1015.
Pharmaceutical Care through Telemedicine of Outpatients with Mood Disorders: Pilot Study
Siqueira NG1, Lago STL1, Fernandes MR2, Rascado RR1, Silva LC3 and Marques LAM1*
1Faculty of Pharmaceutical Sciences, Federal University of Alfenas, Brazil
2University José do Rosário Velano, Brazil
3Pharmaceutical, Brazil
*Corresponding author: Marques LAM, Federal University of Alfenas, Street Gabriel Monteiro da Silva, 700. Alfenas, MG, Brazil
Received: August 22, 2014; Accepted: Sep 06, 2014; Published: Sep 08, 2014
Abstract
Mood disorders represent a major public health problem because they are highly incapacitating, causing social, Physical and personal limitations. Among these, the most prevalent is depression. Pharmaceutical care when in the context of mental health is of great value because it is designed to improve adherence to the treatment and minimize side effects, as well as checking and correcting drug interactions. This practice allied to telemedicine might be an alternative to reach much of the population, with relative convenience. The aim of this study is to evaluate the effectiveness of pharmaceutical care through pharmacotherapy follow-up in depressive symptoms in women who use psychotropic medications, especially those diagnosed with depression. Patients were followed for six months through meetings every two months and telephone calls every month. The research instruments used were pharmacotherapeutic follow-up according to the Dader method, the PHQ-9 depression questionnaire and a questionnaire to measure patient adherence to treatment. The monitored patients were on average 53 (30-74) years old. Most of them, 77.23%, have nine years of study, i.e. incomplete primary education. Over half of the patients (54.55%, n=12) reported that the first episode of mental disorder had occurred more than 10 years ago, however only 25% (n=3) underwent treatment for a period longer than or equal to 10 years. At first only 36% of patients had treatment adherence. At the end of 6 months, the study showed that approximately 73% of patients adhered to treatment and 27% reported at least one treatment interruption. The study also showed positive influence on depression symptoms with a statistically significant decrease of them (p<0.0001). The results showed that the developed pharmaceutical care through telemedicine was effective in reducing levels of depression in the studied population.
Keywords: Depression; Pharmaceutical care; Pharmacotherapeutic followup; PHQ-9
Abbreviations
PHQ-9: Patient Health Questionnaire; DRP: Drug-Related Problems; DNO: Drug-Related Negative Clinical Outcomes; CAPS: Psychosocial Care Center Alcohol and Drugs; SSRIs: Selective Serotonin Reuptake Inhibitors; MGT: Morisky-Green Test; ICD-10: International Classification of Diseases
Introduction
Psychological illness is easily perceived because in general individuals who have become ill present behaviors which are considered nonstandard and which are not normally accepted by society. As a result they suffer social exclusion which is summarized in isolation of patients who are not accepted by society [1].
Depression and disorders caused by use the alcohol and psychoses are among the 20 leading causes of disability worldwide. In all regions, neuropsychiatric conditions are the most important causes of disability, and represent about one third of lost years due to disability (years lost due to disability) among adults aged 15 or over [2], mainly in industrialized countries. As a result of the direct effects of the disease on physical functioning we can associate high morbidity and mortality rates as well as high rates of suicidal thoughts and suicide. The social impact of mood disorders is sometimes overlooked but can be important, leading to the isolation of the patient [3].
The use of antidepressant drugs for the treatment of depression has increased significantly since they produce an improvement in depression of 60-70% within a month, whereas with a placebo there is only 30% improvement. This result is barely achieved with other therapeutic approaches except in electroconvulsive therapy [4].
It was shown that continued antidepressant treatment for 6 months reduces the risk of relapse. A meta-analysis showed that patients receiving antidepressants for a period of 2 to 6 months after complete remission of symptoms have reduced risk of relapse in 50% compared to those who took the placebo [5]. However, it is estimated that approximately 1/3 of patients discontinue antidepressant treatment in the first month, and approximately 45% do not exceed the third month of treatment [6].
Factors that may influence non-adherence to treatment are related to the patients, to the disease, to the treatment, to the health professionals and the social environment. Attitudes and beliefs of patients about depressive illness and its treatment are also related to adherence/non-adherence to treatment. Manber et al. [6], by applying the Patient Perception Questionnaire about Depression, observed that how depressed individuals perceive the depressive illness may be useful in predicting preference for a particular type of treatment, expectations about the effectiveness of treatment, adherence and response to treatment [7].
Adherence can be improved when patients and their families are informed about their mental illness and its treatment. Adequate support for patients can restrict the chronicity of the disease, reduce treatment costs, and the number of hospitalizations, and also can improve the quality of life of patients [8].
Antidepressant drugs are the first choice for the treatment of depression. However, sometimes patients may present Problems Related to Drugs (DRP) or Drug-related Negative clinical outcomes (DNO), which may reflect the effectiveness and safety of the treatment [9].
The integration of Pharmaceutical Care (PC) in mental health services can contribute significantly to the increase in adhesion to the treatment, the management of Adverse Drug Reactions (ADR), the prevention or solution for drug interactions and patient, family, and caregiver education [10].
Telemedicine has been defined as the use of electronic information and communication technologies to provide and support health care when distance separates the participants. The technologies used in telemedicine include video conferencing, telephones, computers, Internet, fax, radio and television [11]. Until the present moment there are few studies relating to depression, pharmaceutical care and telemedicine [12]. Pyne et al. [12], in a randomized controlled trial by a team of mental health care consists of nursing, clinical pharmacist and psychiatrist evaluated the intervention through telemedicine. The results were effective but the cost-effectiveness ratio was high. Positive results obtained through telemedicine for patients with mental disorders have also been reached in other studies [13-15].
Thus, the aim of this study was to evaluate the effectiveness of pharmaceutical care in pharmacotherapy follow-up based on the Dáder methodology, using telemedicine as a strategy among patients with a diagnosis of depression at the Alzira Velano Hospital Outpatient Clinic situated in Alfenas, Brazil.
Methods
Study site and period of data collection
The study was conducted at the Alzira Velano Hospital Outpatient Clinic-UNIFENAS in Alfenas, Brazil. The outpatient clinic provides care in various specialties, including psychiatry and psychology. The average flow of care is 80 patients per week. In this outpatient clinic there is no pharmacy service with dispensing of medications. Each patient with depression is seen by the psychiatrist at least three times in the first months of treatment (1 appointment, a return in 40 days and 2 months after the first return). Data collection for the survey was conducted by the researchers from January 2012 to March 2013.
Study sample
Patients selected were those with a diagnosis of depression based on diagnostic criteria of the ICD-10 (F32-F33), seen or treated in the outpatient clinic and who were sent by a psychiatrist to the pharmaceutical care service.
- Criteria for patient inclusion or exclusion from the study.
Female patients, over 18, in the initial phase of treatment (first treatment) or who had received the prescription of a new antidepressant were included. The following were considered as exclusion criteria: medical records of dependency on psychoactive substances, the statement of a diagnosis of schizophrenia, no phone contact, and presence of obvious cognitive impairment that might interfere with the completion of the research instruments.
Experimental design
Twenty two patients with a diagnosis of depression who initially responded to the PHQ-9 depression questionnaire, the adherence questionnaire, and the questionnaire based on the Dáder method were selected. Each questionnaire was answered on an agreed schedule (date and time of the day) in the patient’s home. Follow-up was performed with an initial visit, then two telephone calls – one in the first month and another in the 2nd month after enrollment, followed by another visit 3 months after enrollment, and then two more phone calls – one in the fourth and another in the fifth month after enrollment, and a last visit at the end of the study, completing a 6 month follow-up.
Ethical aspects
Before beginning any procedure, the present study was submitted to the Research Ethics Committee of the Federal University of Alfenas (UNIFAL) and approved under number: 193.680. Each subject was first notified in writing of the voluntary nature of the participation in the study, of being free to stop it at any time without losing monitoring and medical treatment provided by the outpatient clinic, of the performed procedures, of the risks involved, and the use of confidential information that has been collected. Those who agreed to participate in the study signed a consent form.
Procedures
Pharmacotherapy follow-up: The procedure adopted for pharmacotherapy follow-up was based on the Dáder method [9]. The method was initially applied to twenty patients in the intervention group over six months. For ethical reasons there was no control group, the studied group was the control.
Once the consent form was signed the first meeting was scheduled and the date and time agreed with the patient. The patient was asked to put into a bag all existing drugs in his/her household, prescriptions, and recent test results, with the objective of writing the pharmacotherapeutic history of each patient. In addition, the patient responded to the PHQ-9 depression questionnaire, a questionnaire requesting information such as education, occupation, age and other information, and also the adherence questionnaire. The interviews were conducted in the patients’ homes.
The objective of this phase of the Dáder method is to obtain all the possible information of each medication. Thus, the pharmacist may qualify the treatment adherence and the patient’s knowledge of the medications he/she is taking. At the end of pharmaceutical consultation a second contact was scheduled, this time by phone.
All individuals in the sample were submitted to the PHQ-9 depression questionnaire conducted every three months during two meetings to evaluate the effectiveness of the treatment. In this study, the questionnaire was completed by the researcher, not by the patients themselves.
The data were organized and compiled on situational registration cards and then analyzed. Once the DRP (drug-related problems) and DNO (drug-related negative clinical outcomes) were identified, the data were classified into categories according to the classification criteria of the Third Consensus of Granada [16-18].
After identifying the DRP and DNO, patients were properly informed about the occurrence, and after agreement between both parties pharmaceutical intervention was carried out communicating either orally and/or in writing between the pharmacist-patient and the patient-physician-pharmacist. All interventions were recorded.
The third contact by phone also aimed to assess the result of the intervention. The pharmaceutical intervention consisted of actions that aimed at the resolution of DNO, and also health education and advice on hygiene and dietary habits – either orally and/or in writing.
The evaluation of the results of the pharmaceutical interventions was performed after a pre-set period with each user, and whether the DNO was solved or not was also checked. When the evaluated parameter showed the effectiveness of drug treatment for depression, the PHQ-9 depression questionnaire was used.
The other visits and phone calls aimed at monitoring the performed interventions and patient assessment. In the second and third visits, after 3 and 6 months follow-up respectively, treatment adherence questionnaires and the PHQ-9 for depression were again applied.
Evaluation of adherence to treatment of the medication users: The development of the questionnaire that addresses the patient’s adherence to treatment was based on traditional methods of selfreporting of non-adherence [19]. This questionnaire was applied to the study group before and after pharmacotherapeutic follow up in order to assess whether there was an improvement in the levels of adhesion.
One of the methods used to assess adherence to drug therapy was the method of Morisky, Green and Levine [19], also known as the Morisky method, who developed a scale to measure adherence using questions for the patient. The scale consists of four “yes” or “no” questions of which the answers are used to determine whether treatment failure can be attributed to forgetfulness, carelessness, occurrence of the general improvement of the patient or adverse reactions caused by the drug. The questions were as follows: ‘In the last month have you forgotten to take your medicine?’; ‘In the last month even when remembering, have you stopped taking your medicine?’; ‘In the last month have you ever stopped taking your medicine when you felt better?’; and ‘In the last month have you ever stopped taking your medication when you did not feel well?’.
The questions are designed in such a way as to minimize the deviations of responses, mostly positive. The adherence level is considered high when the number of yes responses is zero (4 points). An average level correlates with one or two yes answers (3 and 2 points) and the low level refers to when the patient responds to three or four positive responses (1 and 0).
Results and Discussion
Description of the studied population
In this study we performed pharmacotherapeutic follow up of 22 female patients who had been diagnosed with depression and had been attended to at the Vila Esperança outpatient clinic, thus making the drawing up of the socioeconomic profile possible (Table 1).
Characteristics
N
%
Marital Status
Single
3
13.64
Married
16
72.73
Divorced
1
4.55
Widow
2
9.09
Education
Less than 9 years of schooling
17
77.27
More than 9 years of schooling
5
22.73
Religion
Catholic
17
77.27
Evangelical
3
13,64
Other
2
9.09
Occupation
Housewife
14
63.64
Retired
3
13.64
Other
5
22.73
Table 1: Socioeconomic profile of the studied population.
The patients in this study are on average 53 (30-74) years old and 68.18% (n = 15) are between 30-50 years old, and 31.82% (n = 7) between 60-74 years and therefore elderly as defined by the Elderly Statute. The majority of them, 77.23%, have nine years of study, i.e. incomplete primary education.
According to Castro and Colet [20], depression mostly affects women and patients aged between 51 and 60 years old and the mean age of the patients of this study was within this range (53 years old). This result can also be observed in a study by Chellappa and Araújo [21], which was conducted with patients diagnosed with depression treated at the outpatient clinic of psychiatry, University Hospital Onofre Lopes in Natal.
Still comparing the results with those obtained by Castro and Colet [20], most patients in this study were married, which contradicts the work of Gazalle et al.[22], who reported that individuals who do not have partners or not living with their spouse were more affected with depressive symptoms.
The study performed by Oliveira and Freitas [23], which examined the socioeconomic profile of patients seen at CAPS Queizeramobin in Ceará, points out that 55% of patients had only primary education, a result also observed in this study, where 77.27% of the group analyzed had less than nine years of schooling.
In the present study we found that most patients were unemployed (77.28%), a result consistent with those observed by Castro and Colet [20], in which 57.52% of patients were in the same situation.
Over half of the patients (54.55%, n = 12) reported that the first depressive episode had occurred more than 10 years ago, however only 25% (n = 3) of them underwent treatment for a period equal to or longer than 10 years. When questioned about the use of drugs, only 3 patients reported having difficulty in managing the drugs.
The majority of patients (86.36%, n = 19) believed that depression can be controlled with medication and/or diet, but 63.16% (n = 12) of them said that depression is a disease for a lifetime and cannot be cured.
Drug treatment profile of the studied population
On the first visit the average number of drugs used by the patients was 5.32 of which 1.41 were drugs for depression treatment, anxiety or insomnia, and the remaining were used for hypertension, diabetes mellitus and other comorbidities treatment. On the last visit the mean value of medications per patient was 5.23 and 1.32 for antidepressants. So, it was noticed that there was no significant difference in the amount of drugs when relating the two occasions (p = 0.3943).
The average treatment time was 4.64 years, ranging from 1 to14 years. Concerning the time at which the first episode occurred and the time of depression treatment, only 3 patients with over 10 years of depression received treatment from the onset of symptoms.
Considering the prescribed classes of medication, it was observed that most drugs used are Selective Serotonin Reuptake Inhibitors (SSRIs), tricyclic antidepressants and benzodiazepines (Table 2). Among these, SSRIs represent the most widely used drug class among the study population.
Medications
N
%
Selective Serotonin Reuptake Inhibitors
12
35.29
Benzodiazepines
11
32.35
Tricyclic antidepressants
8
23.53
Other psychotropics
3
8.82
Table 2: Drugs for depression used by patients at the start of the study.
This result was also reported by Marques et al. [18], where the SSRI drugs had the highest percentage of prescriptions (60%), followed by benzodiazepines (32%) and tricyclic antidepressants (28%).
Assessment of treatment adherence by the Morisky-Green test
To evaluate adherence of the population studied we applied the Morisky-Green Test (MGT) [19]. Patients were grouped according to the score, either 0, 1, 2, 3 or 4, wherein each question gets a point if the answer is negative. High adherence was defined as 4 points scored, mean adherence 2-3 points scored and low adherence for those with scores equal to 1 and zero.
At the beginning of this study only 36% of patients had treatment adherence. At the end of 6 months the study showed that approximately 73% of patients adhered to treatment and 27% reported at least one treatment interruption.
In a face to face study, Bultman and Svarstad [24], who conducted interviews with patients to evaluate the drug history, knowledge of and belief in the drug, patient satisfaction and medication adherence, the rate of medication adherence was 76% within 3 months. The remaining 24% reported only one interruption of the treatment during the same period. In our study it was observed that pharmacotherapeutic follow up positively influenced adherence of patients taking the antidepressant for the first time.
The number of patients with high adherence increased by 50% at the end of the study compared to the beginning. The outcome of patients regarding compliance was also considered individually (Table 3).
Beginning
Conclusion
Evolution
N
Low
Maintained
1
Low
Average
Increased
3
High
Increased
2
Low
Reduced
1
Average
Average
Maintained
1
High
Increased
6
Low
Reduced
0
High
Average
Reduced
0
High
Maintained
8
Table 3: Patient evolution according to treatment adherence.
According to patient’s evolution, it could be observed that 50% (n = 11) had an increase in adherence and 45.5% (n = 10) maintained the initial adherence.
In a review article, Al-Jumah et al. [25] found that five of the six studies that included telephone contact with patients for intervention, reported a substantial improvement in medication adherence.
Since the interventions were made in person or via telephone it was possible to achieve a significant increase in the number of patients abiding to the therapy. The pharmacist becomes an important figure in adherence to treatment of patients because he/she is close to the patient and works primarily with guidance, educating on the importance of the correct use of drugs and increasing adherence.
According to the guidelines of the Brazilian Medical Association for the treatment of depression, treatment should be performed in three phases, the acute phase, the continuation phase and the maintenance phase. The duration of treatment in the acute phase ranges from 2 to 3 months, during the continuation phase for 4 to 6 months and during maintenance phase 5 years or so[6]. The treatment time, as well as its complexity and side effects are important factors in patient adherence [7] and therefore increase the need for pharmaceutical monitoring and intervening for adherence to proper treatment.
Compliance with therapy is important because incorrect use of drugs can lead to recurrence and then the chance that the next antidepressant treatment function decreases with each new attempt [6].
Assessment of depressive symptoms
With the application of the PHQ-9 questionnaire, the level of depression in the population at the beginning and end of the study can evaluated. The normality of the data was performed using the Shapiro-Wilk test and was considered normal when p is greater than or equal to 0.05. For normal data, the paired t-test was used and for non-normal the Wilcoxon test was used, both to assess the significance of the data (p unilateral or less than 0.05).
Regarding depressive symptoms, there was a reduction of the median score of PHQ-9 from 10.5 +-6.04 to 3.0 +-4.69 (p <0.0001). It was noticed that there was a reduction in the score on both measuring instrument and therefore a significant reduction in depressive symptoms.
Also comparing the scores at the beginning and at the end of the study, 90.90% (n = 20) of patients experienced a decrease in depressive symptoms.
In a study by Finley et al. [3] using the PHQ-9 as a means of assessing the severity of depressive symptoms, 80% of patients had a reduction in the score between the initial and the last visit of the follow-up, 8% had no change in the severity and 12% of patients had a worsening. The average score was 11.5 ± 6.6 initially and at the last follow-up was 5.3 ± 4.7 (p <0.0001), a similar result to that obtained in this study.
Most patients had some indicator of depression, and from these most had Indicators for mild major depression, followed by moderate-severe major depression on the first visit. After the end of follow-up the number of patients with no indicators of depression was 16. Remission of depressive symptoms was therefore approximately 59% (n = 13).
Quantification of the problems related to drugs, negative outcomes associated with medications, and pharmaceutical interventions
The Problems Related to Medicines (DRPs) are defined as health problems and negative clinical outcomes, accrued from pharmacotherapy, that are produced by various causes and lead to failure to achieve the therapeutic effect or to the appearance of unwanted effects [16].
With the review of pharmacotherapy and becoming acquainted with the condition of each patient, 32 DRPs were detected in 82% of patients, of whom 81.3% were resolved by pharmacist intervention. The most frequent DRP was related to doses, prescription and/ or inadequate duration, followed by non-adherence (Figure 1). Similarly, Marques et al. [18] characterized these two DRPs as the most frequent, relating the cases of non-compliance with the attitude of the patient (not voluntary adherence).
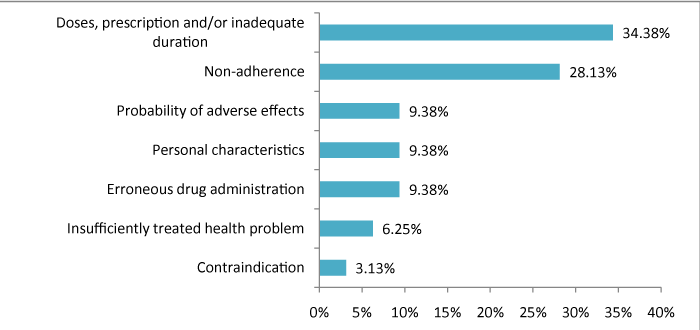
Figure 1: DRPs identified in patients this study.
In cases of non-adherence, 88.9% were solved with guidance on medicines, the importance of utilizing information about the disease, organizing medications and the use of pictograms and/or explanatory pictures.
Drug-related negative clinical outcomes (DNOs) are “health problems, unwanted changes in the health status of the patient due to the use (or misuse) of drugs. To measure them a clinical variable was used (symptom, sign, clinical event, metabolic or physiological measurement, death), which does not reach therapeutic goals established for the patient” [9].
A total of 27 DNOs were detected in 77% of patients. Of these, 77.8% were solved with the interventions. The most frequent DNO was quantitative ineffectiveness followed by quantitative insecurity (Figure 2).
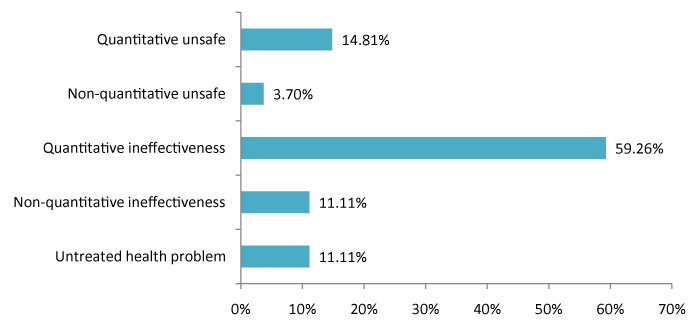
Figure 2: DNOs identified in patients this study.
During follow up when a DRP or DNO was detected the pharmacist intervened to solve these problems. Interventions were between the pharmacist, the patient and/or psychiatrist. While necessary, the doctor was contacted to discuss each patient’s case in order to avoid further problems and solve existing ones.
From a total of 54 interventions, 2.5 per patient on average, more than half (55.6%) were on patient education on how to use and manage the medications, on increasing adherence and on non pharmacologic actions (Table 4). This result suggests that the pharmacist can intervene independently, without the need to contact the prescriber, with positive effects on the health of patients. Interventions with prescribers were also important and most were to modify the drug dose or adding a drug to the treatment.
Pharmaceutical Intervention
Percentage (%)
Change dose
22.22%
Change posology
3.70%
Add medication
12.96%
Replace medication
5.56%
Mode of use and administration
7.41%
increasing adherence
18.52%
Nonpharmacologic actions
29.63%
Table 4: Quantification of pharmaceutical interventions.
The results demonstrated that the pharmaceutical care was effective in reducing levels of depression n this population (p <0.0001). Also, that is possible to run pharmacotherapeutic follow up through in-person visits and telephone contacts. This facilitates the pharmacist’s job, saving resources and enabling patient access when residing in regions far from the city center for pharmacotherapy follow-up.
Furthermore, this study evidences that the pharmacist was able to identify and resolve DRPs and DNOs that occurred due to the use/nonuse of drugs. For this, the pharmacist used Interventions, both in writing or orally, with the patient and the prescriber. Most interventions were performed with the patient and did not require any contact with the doctor. Where physician intervention was needed it was well accepted because there was a channel of information between these health professionals, facilitating actions.
One of the limitations of this pilot study was not having a control group and using a small number of participants, because randomized controlled studies have been highly scientific. Another important limitation is that the follow-up time was relatively short compared with other studies. Eventually the goal is to expand the study to a larger number of patients, include a control group and perform pharmacotherapeutic follow-up by 12 months.
Conclusion
This study suggests that together the pharmacist and the doctor are important to the care of patients with depression by increasing adherence to treatment and consequently, the effectiveness and safety, minimizing depression symptoms. Furthermore, we could detect and solve most DRPs and DNOs present in the study population. The results demonstrated that pharmaceutical care coupled with telemedicine, was effective.
Financial Supports: PROEXT/MEC/SESU – 2014.
References
- Spadini LS, de Mello e Souza MC. [Mental illness in the view of patients and their families]. Rev Esc Enferm USP. 2006; 40: 123-127.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global burden of disease and risk factors. New York: Oxford University Press. 2006.
- Finley PR, Bluml BM, Bunting BA, Kiser SN. Clinical and economic outcomes of a pilot project examining pharmacist-focused collaborative care treatment for depression. J Am Pharm Assoc (2003). 2011; 51: 40-49.
- Souza FGM. Tratamento da depressãão. Rev Bras Psiquiatr. 1999; 21: 18-23.
- Fleck MPA, Lafer B, Sougey EB, Porto JA, Brasil MA, Juruena MF. Diretrizes da associação médica brasileira para o tratamento da depressão. Rev Bras Psiquiatr. 2003; 25: 114-122.
- Manber R, Chambers AS, Hitt SK, McGahuey C, Delgado P, Allen JJ. Patients' perception of their depressive illness. J Psychiatr Res. 2003; 37: 335-343.
- Cunha MF, Gandini RC. Adesão e não-adesão ao tratamento farmacológico para depressão. Psic Teor e Pesq. 2009; 25: 409-418.
- Thase ME. The clinical, psychosocial, and pharmacoeconomic ramifications of remission. Am J Manag Care. 2001; 7: S377-385.
- Hernández DS, Castro MMS, Dáder MJF. Método Dáder: guía de seguimiento farmacoterapéutico. Grupo de Investigación en Atención Farmacéutica de la Universidad de Granada. 2007; 3.
- Fridman GA, Filinger EJ. Atención farmacéutica en pacientes ambulatorios con trastornos psiquiátricos. Lat Am J Pharm. 2003; 22: 351-354.
- Angaran DM. Telemedicine and telepharmacy: current status and future implications. Am J Health Syst Pharm. 1999; 56: 1405-1426.
- Pyne JM, Fortney JC, Tripathi SP, Maciejewski ML, Edlund MJ, Williams DK. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010; 67: 812-821.
- Fortney JC, Pyne JM, Mouden SB, Mittal D, Hudson TJ, Schroeder GW, et al. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: a pragmatic randomized comparative effectiveness trial. Am J Psychiatry. 2013; 170: 414-425.
- Fortney JC, Pyne JM, Edlund MJ, Robinson DE, Mittal D, Henderson KL. Design and implementation of the telemedicine-enhanced antidepressant management study. Gen Hosp Psychiatry. 2006; 28: 18-26.
- Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007; 22: 1086-1093.
- Consensus Commitee. Terceiro Consenso de Granada sobre Problemas relacionados con medicamentos (PRM) y Resultados negativos asociados a la medicación (RNM). Ars Pharm. 2007; 48: 5-17.
- Garcia-Jimenez E. Incumplimiento terapéutico como causa de problemas relacionados con medicamentos en el seguimiento farmacoterapéutico [Dissertation]. Universidad de Granada. 2003.
- Marques LAM, Galduróz JCF, Fernandes MR, Beijo LA, Noto AR. Drug-related problems, drug-related negative clinical outcomes and pharmacist interventions in outpatients with depressive disorder. Journal of Scientific Research in Pharmacy. 2014; 3: 26-30.
- Morisky DE, Verde LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986; 24: 67-74.
- Castro ALFM, Colet CF. Perfil socioeconômico e características da depressão de usuários do Centro de Atenção Psicossocial (CAPS) de Panambi/RS. Rev Contexto e Saúde. 2011; 10: 401-408.
- Chellappa SL, Araújo JF. Transtornos do sono em pacientes ambulatoriais com depressão. Revista de Psiquiatria Clínica. 2006; 35: 233-238.
- Gazalle FK, Lima MS, Tavares BF, Hallal PC. [Depressive symptoms and associated factors in an elderly population in southern Brazil]. Rev Saude Publica. 2004; 38: 365-371.
- Oliveira CPA, Freitas RM. Aspectos da atenção farmacêutica no Centro de Atenção Psicossocial do município de Queixeramobim. Infarma. 2008; 20: 24-26.
- Bultman DC, Svarstad BL. Effects of pharmacist monitoring on patient satisfaction with antidepressant medication therapy. J Am Pharm Assoc (Wash). 2002; 42: 36-43.
- Al-Jumah KA, Qureshi NA. Impact of pharmacist interventions on patients' adherence to antidepressants and patient-reported outcomes: a systematic review. Patient Prefer Adherence. 2012; 6: 87-100.