
Research Article
Ann Depress Anxiety. 2014;1(6): 1027.
The Associations of Sleep Factors and Emotional Distress with Cortisol Awakening Response in Outpatients with Major Depressive Disorder
Hsiao FH1, Wang KC2, Yang TT3, Lai YM4, Chen YT4 and Jow GM5*
1Department of Nursing, National Taiwan University, Taiwan
2Department of Health Promotion and Health Education, National Taiwan Normal University, Taiwan
3Department of Psychiatry, National Defense Medical Center, Taiwan
4School of Nursing, Chang-Gung University, Taiwan
5School of Medicine, Fu-Jen Catholic University, Taiwan
*Corresponding author: Jow GM, School of Medicine, Fu-Jen Catholic University, No 510 Chung-Cheng Road, Hsin-Chuang Dist, New Taipei City 24205, Taiwan
Received: September 16, 2014; Accepted: October 10, 2014; Published: October 24, 2014
Abstract
Objectives: The associations of time of awakening, total time slept, anxiety, and depressed levels with awakening cortisol levels and Cortisol Awakening Response (CAR) were compared between outpatients with Major Depressive Disorder (MDD) and healthy control patients.
Methods: Self-reports of depressive and anxiety levels, time of awakening, total time slept, and healthy behaviors were completed by 125 outpatients with Major Depressive Disorder (MDD) and 107 healthy control patients. Saliva samples were collected for all patients to measure cortisol levels at awakening, 30-45 minutes after awakening, and at 12:00, 17:00, and 21:00 hours.
Results: There was no significant difference in CAR (increased cortisol levels from waking to 30-45 minutes post-waking) between MDD outpatients and healthy control patients. However, factors associated with the magnitude of CAR were different between the two groups after controlling for potentially confounding factors such as gender, age, and smoking, alcohol, and coffee habits. Higher awakening cortisol levels were related to early time of awakening in healthy control patients but were correlated instead with higher anxiety levels in MDD outpatients. The larger magnitudes of CAR associated with later time of awakening in healthy subjects, on the other hand, were related to lower anxiety levels and history of mental illness in MDD outpatients.
Conclusion: The time of awakening is likely a normal regulatory factor of morning cortisol responses in healthy people. Rather than this normal regulatory function, anxiety symptoms and family history of mental illness were the main regulatory factors for awakening cortisol levels and CAR magnitude in MDD outpatients. This might play an important role in underlying pathophysiological processes leading to HPA axis dysfunction in this patient group.
Keywords: Major depressive disorder; Anxiety; Sleep; Cortisol Awakening Responses (CAR)
Introduction
An abnormal psychobiological stress response manifested in the Hypothalamic-Pituitary-Adrenal (HPA) axis plays an important role in the development of Major Depressive Disorder (MDD) [1,2]. The Cortisol Awakening Response (CAR) is regarded as a reliable indicator of detecting changes of HPA axis function [3]. The normal CAR shows that cortisol levels reach to peak shortly at 30-45 minutes post waking [4]. A blunted CAR (a lower rise in cortisol levels after wake-up) was reported in a number of studies. A lower rise of cortisol at 15-30 minutes post waking more likely occurred in MDD inpatients than non-depressed inpatients [1]. A meta-analysis study also indicated that stress reactivity in a blunted CAR pattern was commonly found in outpatients with depression [5]. In a community study, compared with healthy subjects, the rise in cortisol levels after 30 minutes was lower in unmediated young depressed subjects [6,7]. Contrast to findings of blunted CAR in MDD patients, a rapid increase of cortisol levels after waking was found in medication-free patients with MDD at acute depressed stage [8], in the patients with recurrence of major depression [9] and in the remitted depressed patients [10]. The inconsistent results of CAR in depressed patients might urge to explore what factors are associated with enhancing or reducing the CAR among MDD patients.
CAR is the distinct phenomenon in HPA axis activity which reflects the specific processes related to awakening [11,12]. A number of studies found that there was no significant relationship between the time of awakening and awakening cortisol levels in MDD recovery patients [13], acutely depressed outpatients [8] or depressed people in community [6]. Different from the results in MDD patients, early time of awakening associated with higher awakening cortisol levels was evidenced in healthy people [6,12]. A review study [11,14] reported that earlier time of awakening tended to be correlated with larger CAR in healthy population. More studies comparing the associations of awakening cortisol levels and the CAR magnitude with sleeping factors between MDD patients and healthy control are needed to clarify the dysfunction of regulatory of CAR due to major depressive disorder.
No significant associations of awakening cortisol levels and CAR with depressive symptoms were consistently evidenced in MDD patients [8,13]. Anxiety symptoms are commonly expressed among MDD patients but their correlations with awakening cortisol levels and the CAR magnitude in MDD patients are not well examined. The aims of this study were to examine the association of awakening cortisol levels and the CAR magnitude with sleep factors (time of awakening and total time slept) and emotional distress (depression and anxiety) to clarify the main determinants of CAR among outpatients with MDD.
Material and Methods
Subjects and assessments
The 144 patients were recruited from the outpatient department of psychiatry at a general hospital when they met the following inclusion criteria: they were between the ages of 18 and 65 and had a primary diagnosis of Major Depressive Disorder (MDD) assessed by a psychiatrist using a structured Mini-International Neuropsychiatric Interview (M.I.N.I.) in accordance with the criterion of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [15]. Those diagnosed as suffering from organic brain damage, substance use disorders, or disorders of adrenal function (e.g., Cushing syndrome, Addison’s disease, adrenal tumor, pituitary tumor) and patients who are on medications such as hormone therapy were excluded. This study was approved by the Hospital Review Board. The primary medical doctor of the outpatient department of psychiatry provided information about the study to potential subjects. With the permission of patients, psychiatrists provided the researcher with patient contact numbers. The researcher contacted each individual and arranged a personal interview in the researcher’s office.
The 117 healthy subjects were recruited using the snowballing method, through invitation of colleagues, friends, and family members of the researchers when they met the following inclusion criteria: the patients were between 18 and 65 years of age and were healthy at the time of recruitment. Exclusion criteria were 1. Chronic medical illness, 2. Acute infectious disease, 3. Current or recent (within the past year) pregnancy, 4. Use of antidepressant medications, 5. Any standing medication regimens other than oral contraceptives during the previous month and 6. A history of depression, anxiety, or other mental psychiatric diagnosis. Through invitations to colleagues, friends and family members of the researchers, healthy subjects were contacted by a research assistant who explained about this study. After the purposes, risks, and benefits of this study had been explained to them verbally and in writing, the subjects gave their written consent to participate in the study. Demographic data and all measurements were then collected.
This study included the Beck Depression Inventory (BDI) and state of the State Anxiety Inventory (STAI) [16,17]. The 21 items of BDI-II are summed to represent the participant’s depressive symptoms and the scores are classified into four levels of depression: 0-13 Normal; 14-19 Mild; 20-28 Moderate; and 29-63 Severe. The scores of 20-item state of STAI developed by Spielberger et al. [17] are summed to represent the participant’s anxiety, producing a range from 20 to 80; higher scores indicate higher levels of anxiety.
The subjects were asked to report their time of awakening, and total time slept. The subjects provided the information about time of going to bed and time of awakening, which was used to calculate total sleep time. The subjects were asked to respond with if they had a smoking, alcohol and coffee drinking habits at the time they collected cortisol samples. About family history of mental illness, they responded with the questions about “if there was anyone in the family being diagnosed with mental illness and what was your relationship with this affected family member”.
All patients were informed and instructed to collect salivary samples at home using neutral cotton salivette tube (Sarstedt, Germany) collection devices. Each participant collected a sample at each of the following five time points: waking up, 30-45 minutes after awakening, 1200 hours, 1700 hours, and 2100 hours. To focus the results of CAR, the data for samples collected at waking up, 30-45 minutes after awakening, and 2100 hours were reported in this paper. The subjects collected the saliva samples only once in a day. The subjects did not brush their teeth before completing the awakening saliva sampling to avoid contaminating the saliva with blood from micro-injuries in the oral cavity. Moreover, the researchers asked the subjects not to eat before collections at awakening and 30-45 minutes after awakening. For the remaining three samples, subjects did not eat for 30-minutes before collecting samples. Except for the above restrictions, subjects followed their normal, daily routines on the sample collection day. The study found that noncompliance with sampling procedure in adult outpatients is minimal if the collecting sampling procedures were clearly explained to subjects [18]. Therefore, to ensure subjects sampling compliance, the researcher clearly explained to each subject about the importance of collecting sampling according to the time table in order to understand the CAR condition. To enhance compliance, each subject was informed that they would receive their individual cortisol reports after cortisol analysis were completed. Moreover, to assess the subjects’ adherence with the cortisol sampling procedure, they were asked to fill in the actual time of collecting samples in the time sheet which provided with the instructed sampling times. The data would be excluded if sampling time at 45 post-waking was 10 minutes late. The data for 19 in 144 MDD outpatients and the 10 in 117 healthy subjects were excluded due to insufficient cortisol levels for analysis and incorrect collection sampling times. Therefore, the data of 125 MDD outpatients and 107 healthy control patients were included for analysis. Free cortisol was measured using a commercially available immunoassay (IBL, Hamburg, Germany). Inter-assay and intra-assay variations were less than 10%.
Statistical analysis
In this study, to consider different time of awakening among subjects, the individual growth curve model was used in analysis process. We analyzed the effects of clinical variables and confounders on the participant’s salivary cortisol levels with the two-level individual growth curve model, which is a variant of multiple regression models appropriate for the nested structure of data [19- 21]. In the present data, salivary cortisol measurements at the three time points were nested within patients. The two nesting levels were called “measurement level” and “participant level”. At each level, clinical variables and confounders can be added to each model. We used a hierarchical approach to assess the effects of clinical variables (family history, depression, anxiety, time of awakening, and total time slept) on the salivary cortisol levels after controlling for the confounding factors (gender, age, and the smoking, drinking alcohol and coffee habits). These salivary cortisol levels were positively skewed. Therefore, the natural logarithm was used to transform the raw cortisol levels to yield an unscrewed distribution for all of the following analyses. The analyzing processes to examine the specific hypothesis are described in the following:
To analyze the transformed initial cortisol level and cortisol awakening response within patients, firstly, the level-1 model (Measurement Level Model) was used to explore the relationship between the initial cortisol level and the cortisol awakening response from awakening, 30-45 minutes after awakening, to the evening 21:00 within patients in this study. The model (like the regression equation) can be expressed as follows:
(Log Cort)ij = π0j + π1j (Time)ij + εij, where εij ~ N(0, Var(εij)) ------(1)
where (Log Cort)ij is the transformed salivary cortisol levels at the i-th time point (i = 1, …, 3) of the j-th participant ( j = 1, …, 125), (Time)ij represents the time of the day at which (Log Cort)ij is measured, π0j and π1j are the intercept and slope of the j-th participant (here, the intercept represents the initial cortisol levels at awakening by coding Time = 0 for the measured time of awakening), and the εijs are normally distributed error terms with mean 0 and variance Var(εij). In this study, (Time)ij was the only time-varying variable in the level-1 model. Furthermore, it was very possible according to the past studies [1,8] and this present data (Figure 1B) that a quadratic individual growth model was considered and modeled by adding another slope coefficient for the additional quadratic term, (Time)ij2, as follows:
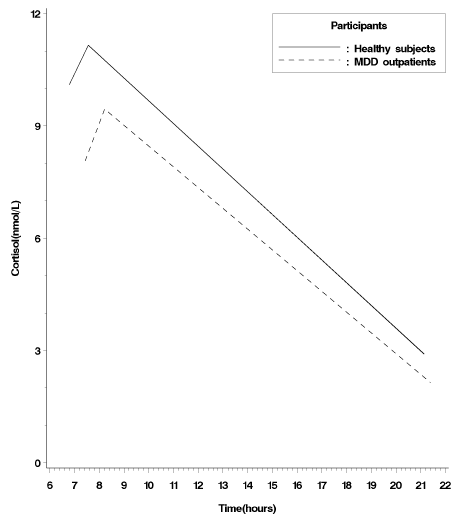
Figure 1B: Observed cortisol awakening response from the participants’ awakening, 45 minutes after awakening, to the evening 21:00 for MDD outpatients and healthy control subjects.
(Log Cort)ij = π0j + π1j (Time)ij + π2j (Time)ij2+ εij, where εij ~ N(0, Var(εij)) ----- (2)
To analyze the differences in the transformed initial cortisol level and cortisol awakening response between patients, the level-2 model (Participant-Level Model) was used to explore between-patients differences and variability in the intercepts and slopes obtained in the level-1 model. The intercept (π0j) and slopes (π1j, π2j) of the j-th participant are allowed to vary across individuals in the level-1 model. The level-2 model without including any clinical variables and confounders is called the unconditional linear (quadratic) model (Equation 3 (4)). In the unconditional model, π0j and π1j (π2j) can be split into two components: a fixed component (β00, β10 (β20)) that is constant across patients (called fixed effect) and a random component (u0j, u1j) that varies across patients (called random effect).
\π0j = β00 + u0j,
π1j = β10 + u1j
, whereπ0j = β00 + u0j,
π1j = β10 + u1j,
π2j = β20, where
In this unconditional model, β00 and β10 (β20) are the intercepts (fixed effects) for the intercept (π0j) and slope(s) (π1j (π2j)) in the level-1 model, respectively; u0j and u1j are the unique effects (random effects) for the j-th participant on the intercept and slope, respectively; and Ωu is a variance-covariance matrix of the random effects. In this study, we fitted the transformed salivary cortisol responses by a twolevel unconditional quadratic model because the added quadratic component did improve the model fit significantly in this model (Linear: -2 Log Likelihood= 1390.4; Quadratic: -2 Log Likelihood= 1358.8; Deviance difference= 31.6, df= 1, adjusted p < 0.0001 by using the Bonferroni adjustments).
To analyze the impacts of clinical variables and confounders on the transformed initial cortisol level and cortisol awakening response, the clinical variables and confounders are considered in this conditional model. The model (called conditional model, Equation 5) can be rewritten as follows,
π0j = β00 + β01(gender)j + β02(age)j +...+ u0j,
π1j = β10 + β11(gender)j + β12(age)j + … + u1j,
π2j = β20,
where,In this conditional model, the individual intercept and slopes (π0j, π1j and π2j) are modeled as the dependent variable with the confounders (gender, age, and the smoking and drinking alcohol and coffee habits). After excluding the effects of the confounders, the hierarchical approach was then applied to evaluate the effects of the clinical variables (family history of mental illness, depression, anxiety, time of awakening, and total time slept) on the salivary cortisol levels.
To compare the results of the cortisol awakening response and associated factors between MDD outpatients and healthy subjects, Figure 1A indicated cortisol levels at time of awakening, 30-45 minutes after awakening, and at 21:00 for MDD outpatients and healthy control subjects. Moreover, a two-level conditional quadratic model (Figure 1B) was used to fit the log-transformed salivary cortisol levels to compare the differences between MDD outpatients and healthy subjects in the initial cortisol level (intercept) and awakening cortisol response (slopes) since the added quadratic component did improve the model fit significantly (Linear: -2 Log Likelihood= 670.9; Quadratic: -2 Log Likelihood= 656.6; Deviance difference= 14.3, df= 1, adjusted p = 0.003 by using the Bonferroni adjustments). Furthermore, the hierarchical approach to the MDD outpatients’ data was also used to analyze the healthy subjects’ data to compare the differences between two groups in the associated factors with the initial cortisol level and the awakening cortisol response. Data analyses were through SAS software, version 9.2.
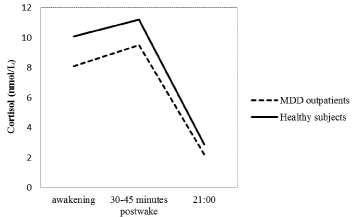
Figure 1A: Cortisol levels at time of awakening, 30-45 minutes after awakening, and at 21:00 for MDD outpatients and healthy control subjects.
To present and display the nature of the interaction for growth curve models, we took the recommendation of Biesanz et al. [22] to use Aiken and West’s [23] procedure to graph the figures: the impact of the factor (Z) (such as STAI anxiety levels, Figure 2) on salivary cortisol (Y) in the time of period (X) (according to the patients’ averaged time of waking up and evening). Moreover, the figure shows the effects of three levels of Z factor on awakening cortisol response (slopes), indicated by at one standard deviation below Z’s mean, at Z’s mean, and at one standard deviation above Z’s mean (Figure 2).
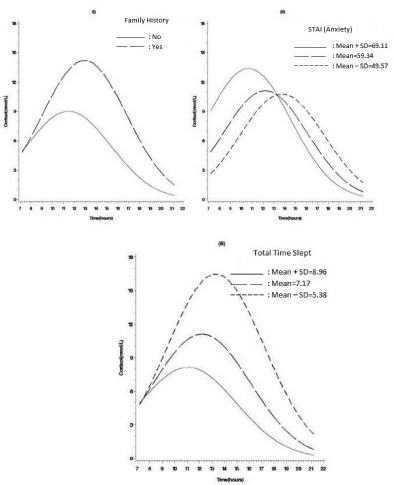
Figure 2: Estimated cortisol awakening response by (i) family history of mental illness, (ii) anxiety levels (STAI), (iii) total time slept in MDD outpatients.
Results
Descriptive statistics of MDD outpatients and healthy control patients
As indicated in Table 1, MDD outpatients and healthy control subjects were primarily female and mainly middle aged. The mean ages for MDD outpatients and healthy control subjects were 40.63±11.09 and 38.31±9.84. There were also no significant differences in alcohol and coffee drinking habits. Nevertheless, compared with healthy subjects, more MDD outpatients had smoking behaviors habitually. Family history of mental illness was significantly more frequent in MDD outpatients than in healthy control patients. Moreover, MDD outpatients had significantly more severe levels of depression than healthy subjects. The average BDI scores showed moderate levels of depression in MDD outpatients, while a normal level was shown in healthy subjects. The mean scores on STAI indicated that MDD outpatients had higher anxiety levels than healthy counterparts. There were no significant differences between groups in total time slept. However, average time of awakening was later in MDD outpatients than healthy subjects. All MDD outpatients were medicated at the time of cortisol sampling. Most subjects received Selective Serotonin uptake Inhibitors (SSRI), including fluoxetine (20-40mg) and sertraline (50-100mg); a minority of patients received serotoninnor epinephrine reuptake inhibitors, including venlafaxine and duloxetine. Another common type of medication taken by patients was hypnotics (yes= 97(77.6%), no=28(22.4%)). Imidazopyridines (Zolpidem) were the most prescribed; other hypnotics used by study patients included Benzodiazepine (Estazolam, Lorazepam, Clonazepam) and Cyclopyrrolones (Zopiclone).
MDD outpatients
(n=125)
Controls
(n=107)
?x2/t(p)
Gender female
male
85
40
69
38
0.319(0.572)
Age
40.63±11.09
38.31±9.84
1.67(0.096)
Family history no
yes
69
51
102
5
43.553(0.000)***
Smoking no
yes
90
35
98
9
14.394(0.000)***
Alcohol drinking no
yes
109
16
95
12
0.137(0.712)
Coffee drinking no
yes
54
71
39
68
1.094(0.296)
BDI
28.10±13.27
7.67±7.50
14.68(0.000)***
Sate of STAI
59.34±9.77
40.61±10.40
14.13(0.000)***
Time of going to bed
24.04±1.52
23.81±1.30
1.25(0.212)
Time of awakening
7.22±1.87
6.70±1.26
2.51(0.013)*
Total time slept
7.17±1.79
6.89±1.22
1.41(0.160)
Cortisol level at awakening (mnol/L)
8.06±5.32
10.1±4.20
-3.23(0.001)**
Cortisol level at 30-45 minutes postwake
9.45±4.41
11.15±4.57
-2.85(0.005)**
Cortisol level at 21:00
2.15±2.18
2.91±1.89
-2.81(0.005)**
* p < 0.05, ** p < 0.01, *** p < 0.001.
Table 1: Descriptive Results of the Two Samples (n, means±SD).
Comparison of the awakening cortisol levels and the CAR between MDD outpatients and healthy control patients
As indicated in Table 1, awakening cortisol levels, 30-45 minutespost wake cortisol levels and cortisol levels at 21:00 were significantly lower in MDD outpatients than in healthy control patients. However, the slopes from waking up to 30-45 minutes post waking were similar between the two groups (β=-0.003, SE= 0.009, t =-0.31, p= 0.756).
In Table 2, for MDD outpatients, higher awakening cortisol levels were related to higher anxiety levels (β = 0.06, SE=0.03, t= 2.09, p =0.039) (Figures 2-ii). By contrast, for healthy control patients, the higher awakening cortisol levels were correlated with early time of awakening (β = -0.25, SE=0.10, t= -2.59, p =0.011) and longer total sleep time (β = 0.15, SE= 0.06, t= 2.34, p = 0.022) (Figures 3-i and 3-ii), suggesting that sleeping factors might have an important role in influencing awakening cortisol levels in healthy subjects but not in MDD outpatients. Moreover, the gender effect on awakening cortisol levels was only found in healthy subjects. Healthy female subjects were more likely than males to have higher awakening cortisol levels (β = -0.15, SE= 0.07, t= -2.29, p = 0.024; Figure 3-iii). Family history of mental illness and depressed symptoms were not related to awakening cortisol levels (p > 0.05) in both MDD and healthy groups.
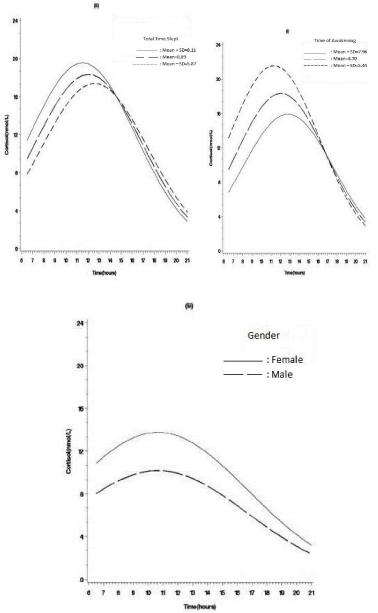
Figure 3: Estimated cortisol awakening response by (i) time of awakening, (ii) total time slept, (iii) gender in healthy control subjects.
MDD Outpatients
Estimate(SE)a
Healthy Subjects
Estimate(SE)a
Model 1
Model 2
Model 3
Model 1
Model 2
Model 3
Fixed effects
Intercept
1.67
(0.17)***
1.66 (0.27)***
1.66
(0.29)***
2.24 (0.05)***
2.09
(0.12)***
2.17 (0.17)***
Gender
0.13 (0.19)
-0.15* (0.07)
Age
0.02 (0.02)
0.01 (0.01)
Smoking
0.08 (0.20)
0.004
(0.11)
Drinking alcohol
-0.11
(0.27)
0.09
(0.09)
Drinking coffee
-0.04
(0.18)
-0.06 (0.06)
Family history
-0.30
(0.18)
0.08
(0.14)
Depression
-0.01
(0.02)
-0.01 (0.01)
Anxiety
0.06*
(0.03)
-0.01
(0.01)
Time of go-to-bed
-0.02
(0.02)
-0.01
(0.01)
Time of awakening
-0.03
(0.09)
-0.25* (0.10)
Total time slept
0.07
(0.12)
0.15* (0.06)
Timeb
0.29***
(0.08)
0.31***
(0.08)
0.31**
(0.09)
0.03
(0.04)
0.30** (0.11)
0.33*
(0.16)
Gender
-0.01
(0.02)
0.004
(0.01)
Age
-0.002
(0.002)
-0.001 (0.001)
Smoking
-0.05
(0.03)
0.02
(0.01)
Drinking alcohol
0.02
(0.03)
-0.01
(0.01)
Drinking coffee
0.02
(0.02)
0.0003
(0.01)
Family history
0.05*
(0.02)
0.001
(0.02)
Depression
0.004
(0.002)
0.001
(0.001)
Anxiety
-0.01**
(0.003)
-0.0001 (0.001)
Time of go-to-bed
0.002
(0.003)
0.001
(0.001)
Time of awakening
0.02
(0.01)
0.03*
(0.01)
Total time slept
-0.04**
(0.01)
-0.02*
(0.01)
a SE represents the standard error of the estimate, b Time was centered about the mean of time of awakening, * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 2: Effects of Confounders and Clinical Variables on the Initial Cortisol Level and the Cortisol Awakening Response in MDD Outpatients (n=125) and healthy subjects (n=107).
For MDD outpatients, Table 2 showed that anxiety levels were negatively related to magnitude of CAR (β = -0.01, SE = 0.003, t = -3.11, p = 0.002). As shown in Figure 2-ii, Figure 2-iii the results indicated that higher anxiety levels tended to show a lower rise of CAR. There was a positive correlation between family history of mental illness and magnitude of the CAR (β = 0.05, SE = 0.02, t = 2.33, p = 0.021). As shown in Figure 2-i, a lower rise at 30-45 minutes post wake more likely occurred in MDD outpatients without than with family histories of mental illness. Different from the findings of MDD outpatients, a lower increase of CAR correlated with early time of awakening (β = 0.03, SE= 0.01, t= 2.53, p = 0.012) was only observed in healthy subjects. There were similarity in the longer total sleep time related to a lower rise of cortisol levels after waking between MDD outpatients (β = -0.04, SE= 0.01, t= -3.11, p = 0.002) and healthy subjects (β = -0.02, SE= 0.01, t= -2.30, p = 0.023). Moreover, depressive symptoms not related to magnitude of CAR were also observed in both groups.
Discussion
This study indicated that awakening cortisol levels, 30-45 minutes post-waking cortisol levels and cortisol levels at 21:00 were significantly lower in MDD outpatients than in healthy subjects. No significant differences in CAR (increased cortisol levels from waking to 30-45 minutes post-waking) between MDD outpatients and healthy subjects were observed. However, factors associated with awakening cortisol levels and the magnitude of CAR were different between the two groups after controlling for confounding factors such as gender, age, and smoking, alcohol, and coffee habits. For MDD outpatients, anxiety levels and family history of mental illness were correlated with awakening cortisol levels and the magnitude of CAR. For healthy subjects, however, time of awakening was the main factor associated with awakening cortisol levels and the magnitude of CAR. This might suggest that anxiety symptoms and family history of mental illness as the regulatory basis for cortisol levels at awakening and magnitude of CAR might be related to pathophysiological processes leading to HPA axis dysfunction in MDD outpatients.
Hypocortisolism (lower awakening cortisol levels, 30-45 minutes post-waking levels, and levels at 21:00) in MDD outpatients might be attributable to the effects of antidepressant drugs that all MDD outpatients had received at the time of cortisol sampling. Antidepressant drugs have been found to have positive effects on normalization of HPA functions through improvement of hypercortisolism or decreasing the reactivity of dexamethasone- CRH [24]. Moreover, antidepressant drugs improve flattened CAR by interacting with Mineralocorticoid (MR) [25]. The effects of antidepressants on CAR might contribute to our finding of no significant difference in magnitude of CAR between MDD outpatients and healthy control patients. However, differences in factors associated with awakening cortisol levels and CAR between MDD outpatients and healthy control patients suggest there might be an impairment of the regulatory factors for HPA axis function in those with major depressive disorder.
Early time of awakening was consistently associated with higher awakening cortisol levels in healthy control patients in the present study and in previous studies [6,11,12]. These results support that time of awakening likely plays a normal regulatory function for awakening cortisol levels in healthy people. This normal regulatory function might be impaired in MDD outpatients, as no significant relationship between the time of awakening and awakening cortisol levels in MDD patients was consistently shown in the present study or previous studies [6,8,13]. Typically, waking up enhances activity in the HPA axis and elevates neocortical networks by activating in brainstem arousal systems [26]. The failure of the HPA axis to respond to time of awakening in MDD outpatients may illustrate the pathological impact of major depression on the timing of the sleepwake cycle in relation to cortisol rhythms.
We found that for MDD outpatients, higher anxiety levels, rather than time of awakening, were related to higher awakening cortisol levels and lower magnitudes of CAR. Similarly, the review study revealed that the greater levels of rumination following a mood induction task were associated with a reduced CAR [11]. Koh [27] proposed that elevated immune function in response to anxiety is the body’s natural response to stress. The physical anticipation of upcoming demands might be related to the regulation of CAR magnitude [14]. A blunted CAR might be seen as a physical adaptation to help individuals from engaging in stress situations [28]. Anxiety symptoms related to awakening cortisol levels and CAR magnitude might illustrate the process of physical response to stress in MDD outpatients.
This study revealed that in addition to anxiety symptoms, the magnitude of CAR was associated with a family history of mental illness in MDD outpatients but not in healthy control patients. Family history of mental illness related to a greater magnitude of CAR might demonstrate there was a genetic vulnerability for deregulation of CAR in MDD outpatients. Young people who had a parent with a history of major depression were found to have an enhanced CAR [29]. The potential impact of family history of mental illness on regulation of CAR might explain the role of genetic vulnerability affecting the HPA axis in MDD outpatients.
The present study revealed that longer total time slept was associated with a lower magnitude of CAR in both healthy control patients and MDD outpatients. The findings are consistent with previous studies on healthy individuals [4] and MDD patients [8]. The results suggest that longer total time slept correlated with a blunted CAR were not likely changed due to major depression. In the present study, longer sleep durations were anything greater than about 7 hours in MDD outpatients (mean = 7.17, mean + SD = 8.96 hours) and in healthy control patients (mean = 6.89, mean + SD = 8.11 hours). A blunt CAR was generally related to oversleep (>8 hours) in both groups. The previous study [30] found that the best survival was found among those who slept 7 hours per night while the increases of mortality hazard were found among people who slept 8 hours or more. The correlation of longer sleep durations with a blunt CAR might provide possible explanations for the association of longer sleep durations and increased mortality. The magnitude of CAR decreased after healthy patients were exposed to experimental noise while sleeping at night [31]. It is possible that oversleep might reflect sleep distress due to stress during the night, which might enhance cortisol levels at night and thus subsequently reduce magnitude of CAR in the morning. More studies need to be done to test this hypothesis.
Consistent with previous studies [8,13], this study indicated that awakening cortisol levels and CAR magnitude were not correlated with depressive symptoms in either group. This suggests that depressive symptoms might be unlikely to play a role in affecting the sleep-wake cycle in cortisol rhythms.
Conclusion
The present study concludes that although there were no significant differences in CAR (increased cortisol levels from waking to 30-45 minutes post-waking) between MDD outpatients and healthy control patients, the factors associated with awakening cortisol levels and the magnitude of CAR were different between the two groups after controlling for potentially confounding factors such as gender, age, and smoking, alcohol, and coffee habits). In MDD outpatients, a lack of correlation between time of awakening and awakening cortisol levels and the magnitude of CAR suggests a lack of adequate regulation of the sleep-wake cycle in cortisol rhythms. Rather than time of awakening, a family history of mental illness and anxiety symptoms were the regulatory factors for morning cortisol responses in MDD outpatients. Studying factors associated with awakening cortisol levels and CAR might be a reliable method for studying the pathophysiological mechanisms of HPA axis function in major depression. Future studies should take into account the limitations of this study, by including use of a longitudinal design and collecting more detailed information about sleep distress using polysomnography for the assessment of objective sleep parameters and the association with awakening cortisol levels and CAR magnitude. Moreover, due to Body Mass Index is not collected, its impacts on sleep problems which may have influence on cortisol responses are unknown in this study. Despite the limitations of this study, the findings contribute to our understanding of the different factors related to awakening cortisol levels and magnitude of CAR between MDD outpatients and healthy control patients, which might play an important role in underlying pathophysiological processes leading to HPA axis dysfunction in MDD outpatients.
References
- Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006; 31: 900-904.
- Rydmark I, Wahlberg K, Ghatan P, Modellb S, Nygrena A, Ingvara M, et al. Neuroendocrine, cognitive and structural imaging characteristics of women on long term sick leave with job stress–induced depression. Biol Psychiatry. 2006; 60: 867-873.
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004; 7: 29-37.
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000; 25: 707-720.
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005; 30: 846-856.
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. J Abnorm Psychol. 2005; 114: 697–705.
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, et al. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biol Psychiatry. 2010; 68: 847-853.
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology (Berl). 2005; 182: 54-57.
- Hardeveld F, Spijker J, Vreeburg SA, Graaf RD, Hendriks SM, Licht CMM, et al. Incrased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendcrinology. 2014; 50: 62-71.
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009; 66: 617-626.
- Elder GJ, Wetherell MA, Barclay NL, Ellis JG. The cortisol awakening response--applications and implications for sleep medicine. Sleep Med Rev. 2014; 18: 215-224.
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007; 32: 358-366.
- Bhagwagar Z, Hafizi S, Cowen PJ. Increase in concentration of waking salivary cortisol in recovered patients with depression. Am J Psychiatry. 2003; 160: 1890-1891.
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009; 72: 67-73.
- Heaton P, Davis RE, Happe FG. Research note: exceptional absolute pitch perception for spoken words in an able adult with autism. Neuropsychologia. 2008; 46: 2095-2098.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4: 561-571.
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory: Test manual. Palo Alto, CA: Consulting Psychologists Press. 1970.
- Remillard AJ, O'Reilly R, Gorecki DK, Blackshaw SL, Quinn D, Keegan DL. The noncompliance factor in the dexamethasone suppression test. Biol Psychiatry. 1993; 33: 755-756.
- Goldstein H. Multilevel Statistical Models. 3rd edn. New York: Oxford University Press. 2003.
- Raudenbush SW, Bryk AS. Hierarchical linear models applications and data analysis methods. Thousand Oaks, CA: Sage Publications. 2002.
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press. 2003.
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psychol Methods. 2004; 9: 30-52.
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage. 1991.
- Zobel AW, Schulze-Rauschenbach S, Von Widdern OC, Metten M, Freymann N, Grasmader K, et al. Improvement of working out not declarative memory is correlated with HPA normalization during antidepressant treatment. J Psychiatr Res. 2004; 38: 377-383.
- Klok MD, Vreeburg SA, Penninx BWJH, Zitman FG, Kloet ERD, DeRijk RH. Common functional mineralocorticoid receptor polymorphisms modulate the cortisol awakening response: Interaction with SSRIs. Psychoneuroendcrinology. 2011; 36: 484-494.
- Steriade MM, McCarley R. Brain Control of Wakefulness and Sleeping. New York: Springer. 2005.
- Koh KB. Emotion and immunity. J Psychosom Res. 1998; 45: 107-115.
- Doane LD, Adam EK. Loneliness and cortisol: momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010; 35: 430-441.
- Mannie ZN, Harmer CJ, Cowen PJ. Increased waking salivary cortisol levels in young people at familial risk of depression. Am J Psychiatry. 2007; 164: 617-621.
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002; 59: 131-136.
- Waye KP, Clow A, Edwards S, Hucklebridge F, Rylander R. Effects of nighttime low frequency noise on the cortisol response to awakening and subjective sleep quality. Life Sciences. 2003: 72: 863–875.