
Editorial
Ann Depress Anxiety. 2014;1(6): 1028.
Prescribing Generic or Branded Antidepressants: What to Choose?
Sultana J1, Parrino F1 and Trifiro G1*
1Department of Clinical and Experimental Medicine, University of Messina, Italy
*Corresponding author: Trifiro G, Department of Clinical and Experimental Medicine, Section of Pharmacology University of Messina, Policlinco Universitario, Via Consolare Valeria, 98125, Messina, Italy
Received: October 28, 2014; Accepted: October 29, 2014; Published: October 30, 2014
Introduction
Antidepressant Drugs (ADs) are one of the most widely used therapeutic classes of drugs. In 2010 antidepressants were the ninth most highly prescribed drug class, with global sales of over $20 billion [1]. The consumption of ADs in European countries has increased significantly between 2007-2011. In Spain the use of ADs has increased by 23%, in Portugal by 20% and in Germany by 46% [2]. Iceland is the country with the highest level of consumption of ADs in the world according to the Organisation for Economic Co-operation and Development (Figure 1) [3].The use of ADs in the US has also increased markedly from $11.2 million in 1998 to $23.3 million in 2010 over the last decade [3].
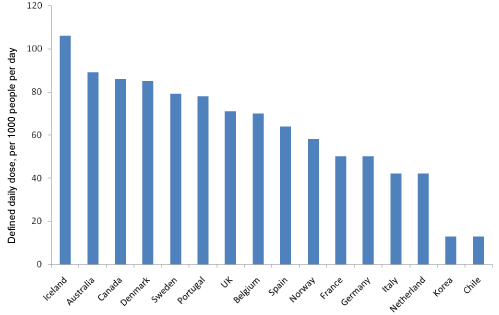
Figure 1: Antidepressant consumption per year (2011) in selected countries around the world. Data was adapted from OECD review (Health at a Glance).
The use of ADs tends to be greater in elderly persons. An American study found that patients aged =65 years were more likely to be in long-term therapy (=2 years) with ADs [4]. A European study investigating the use of psychotropic drugs in six countries (Netherlands, Germany, France, Spain, Italy and Belgium) found that overall, of the non-institutionalized population studied (n=21,425) ADs were the second most commonly prescribed psychotropic drugs (3.7%) after anxiolytics (9.8%). The prevalence of AD use became consistently higher with increasing age; it was highest in the group aged >65 years. Furthermore, comparing the pattern of AD use between different countries it was found that the Netherlands had the lowest prevalence of AD use while Germany the highest prevalence [5].
As with all generic drugs, generic ADs are interchangeable with their brand-name equivalents, but are much cheaper and are manufactured after the innovator company’s patent has expired [6]. SSRIs are the most commonly prescribed AD [7] and several generic ADs are available for some of the most frequently prescribed SSRI such as fluoxetine, (patent expired in 2001) and citalopram (patent expired 2003), which was the most highly prescribed antidepressant on the US market in 2011 followed by sertraline (patent expired 2006) [8]. Escitalopram was one of the most commonly prescribed SSRIs in Italy according to the most recent 2013 data from the Italian Medicines Utilization Monitoring Centre although it was still under patent until some months ago, with a consequent great impact on Italian NHS costs [9].
The bioequivalence between generic and brand-name drugs is established by comparing their bioavailability. This is measured using the Area-Under-the-Curve of the drug concentration–time curve (AUC) and the maximum plasma concentration (C-max) of the drug. By law, the AUC and C-max of a proposed generic drug must be proven to fall within ranges similar to those of the brandname drug for a competent authority to approve the drug’s status as bioequivalent. This means that the bioequivalence of generic to brand-name antidepressants is a necessary prerequisite for generic status, making generic antidepressants pharmacologically and clinically interchangeable with their brand-name counterparts. Several studies have confirmed the bioequivalence of individual generic antidepressants [10-12].
Guidelines of depression treatment suggest that patients should be treated for at least 9-12 months after recovery, based on Institute for Clinical Systems Improvement and World Health Organisation recommendations [13]. Therefore, the long-term use of generics may offer the opportunity to cut healthcare costs. Sustainability in the use of healthcare resources is increasingly prioritised, suggesting that generic antidepressants should be given prominence as a therapeutic option.
The market penetration of generic ADs varies from country to country. The Italian Medicines Utilization Monitoring Centre reports that generic ADs made up almost 40% of all antidepressant prescriptions [9]. In Eastern Europe, the use of generic ADs was found to be 73.6% of all prescribed ADs in 2008 in Croatia but much lower, at 33.5% in Slovenia [14]. Recent findings from the USA show that rates of generic AD use are similar to those in Croatia as of 2010, at 73% [15].However, the struggle for generic antidepressants to penetrate the market to the same degree as brand-name ADs is an ongoing one [16].
The wider diffusion of generic ADs may be hindered by a number of reasons. Patient and prescriber perception of generic drug safety and efficacy is one such reason. A survey administered to approximately 400 psychiatrists in Germany showed that most psychiatrists judge branded drugs as being slightly better than generic drugs with regard to all potential differences (i.e., placebo effect, bioavailability, handling, efficacy) [17]. It is known that patients may perceive generic drugs to be less effective than branded drugs because the packaging or pill colour/shape is different to that of the branded drug [18]. This issue may be more relevant to those patients who are switched over from a branded drug to a generic drug [19]. The prescribing culture specific to physicians of a particular medical speciality is yet another reason which might explain why some prescribers might be more open to prescribing generic drugs. For example, a study by Bolton et al. (2012) suggests that the psychiatrists enrolled in the study were more willing to prescribe generic antidepressants compared to general practitioners. Finally, it is also possible that generic antidepressant companies do not exert the same marketing pressure on prescribers as do the companies of brandname antidepressants. This may be a less investigated aspect of the generic/branded drug utilisation but nevertheless one which could be underestimated as a factor contributing to the lower use of generic antidepressants compared to brand-name antidepressants.
It can be argued that the obstacles hindering the use of generic antidepressants described above do not have a scientific basis. Based on currently known evidence, generic antidepressants are as effective as brand-name antidepressants and much more cost effective [20,21]. Improving patient and prescriber knowledge in this respect can pave the way for more evidence-based prescribing that is increasingly sustainable.
References
- Intercontinental Marketing Services Health Inc (IMS).
- World Health Organisation (WHO).
- Organization for Economic Co-operation and Development (OECD).
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014; 75: 169-177.
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Psychotropic drug utilization in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004; (420):55-64.
- World Health Organisation (WHO).
- Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, Garcia-Cebrian A. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry. 2008; 23: 66-73.
- Intercontinental Marketing Se rvices (IMS) Health Inc Health’s Xponent service.
- Italian National Drug Observatory: Report on Drug Utilisation in Italy. 2013.
- Unterecker S, Proft F, Riederer P, Lauer M, Deckert J, Pfuhlmann B. The comparison of brand-name and generic formulations of venlafaxine: a therapeutic drug monitoring analysis. Ther Drug Monit. 2014; 36: 269-272.
- Gschwend MH, Richter J, Sennewald R, Guserle R, Renner J, Martin W. Bioavailability investigation of two different oral formulations of citalopram, a so-called 'second generation' antidepressant drug. Arzneimittelforschung. 2005; 55: 730-737.
- Jiang T, Rong Z, Xu Y, Chen B, Xie Y, Chen C, et al. Pharmacokinetics and bioavailability comparison of generic and branded citalopram 20 mg tablets: an open-label, randomized-sequence, two-period crossover study in healthy Chinese CYP2C19 extensive metabolizers. Clin Drug Investig. 2013; 33: 1-9.
- Mitchell J, Trangle M, Degnan B, Gabert T, Haight B, Kessler D, et al. Institute for Clinical Systems Improvement (ICSI). Adult Depression in Primary Care. 2013.
- Vitezic D, Kucan M, Vitezic M, Mrsic Pelcic J. Trends in antidepressants and anxiolytics drugs usage in Croatia and Slovenia. 13th Annual European Congress of International Society for Pharmacoeconomics and Outcomes Research (ISPOR); Prag, Ceska. 2010.
- Ventimiglia J, Kalali AH. Generic penetration in the retail antidepressant market. Psychiatry (Edgmont). 2010; 7: 9-11.
- Vlahiotis A, Devine ST, Eichholz J, Kautzner A. Discontinuation rates and health care costs in adult patients starting generic versus brand SSRI or SNRI antidepressants in commercial health plans. J Manag Care Pharm. 2011; 17: 123-132.
- Hamann J, Mendel R, Kissling W, Leucht S. Psychiatrists' decision making between branded and generic drugs. Eur Neuropsychopharmacol. 2013; 23: 686-690.
- Kesselheim AS, Misono AS, Shrank WH, Greene JA, Doherty M, Avorn J, Choudhry NK. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern Med. 2013; 173: 202-208.
- Carbon M, Correll CU. Rational use of generic psychotropic drugs. CNS Drugs. 2013; 27: 353-365.
- Gonzaleza J, Sismeiro C, Dutta S. Can branded drugs benefit from generic entry? The role of detailing and price in switching to non-bioequivalent molecules. International Journal of Research in Marketing. 2008; 25: 247–260
- Colombo GL, Agabiti-Rosei E, Margonato A, Mencacci C, Montecucco CM, Trevisan R. Off-Patent Generic Medicines vs. Off-Patent Brand Medicines for Six Reference Drugs: A Retrospective Claims Data Study from Five Local Healthcare Units in the Lombardy Region of Italy. Plos One. 2013; 8: 82990.