
Research Article
Ann Depress Anxiety. 2014;1(6): 1029.
Affective Network Hyperconnectivity and Hypoconnectivity of Cognitive Control and Ventral Attention Networks in Adults with High Neuroticism Scores
Carballedo A1*, Doyle M1, Lavelle G2, Sojo J1, McCarthy H1, Gormely J2, O’Keane V1 and Frodl T1
1Department of Psychiatry and Trinity College Institute of Neuroscience, Trinity College Dublin, Ireland
2Department of Physiotherapy, St. James’s Hospital and Trinity College Dublin, Ireland
*Corresponding author: Angela Carballedo, Trinity College Institute of Neuroscience, Trinity College Dublin, Ireland
Received: September 29, 2014; Accepted: October 23, 2014; Published: November 01, 2014
Abstract
Introduction: Subjects with high neuroticism are more likely to interpret ordinary situations as negative, and this might contribute to a predisposition toward mood and anxiety disorders. The aim of our study was to determine the localization of neuroticism-related Resting State Functional Connectivity (RSFC) differences between the two groups of high and low neuroticism, and to confirm our hypothesis that subjects with high neuroticism show hyper connectivity in the affective network and hypo connectivity in the cognitive control and attention networks.
Methods: Forty three healthy participants underwent resting state fMRI and completed the NEO Five Factor Personality Inventory. SPM8 and CONN software was used to pre-process and analyse resting state fMRI data. Correlation maps were produced between seed regions of the affective, cognitive control, attention and default mode networks and differences were analysed between groups fully corrected for multiple testing across the whole brain.
Results: Participants with high neuroticism scores displayed significantly greater functional connectivity in the affective network. There was significantly less functional connectivity in the cognitive control network and ventral attention network for participants with high neuroticism scores when compared to those with low neuroticism scores. Discussion: Affective network hyper connectivity might be related to emotional problems or mood disorders that are associated with high neuroticism. Additionally, the hypo connectivity seen in the cognitive control network might have to do with inattention and cognitive deficits that have consistently been found in major depression and anxiety disorders. Thus, oversensitivity in affective systems and at the same time reduced cognitive control might be in line with increased stress sensitivity and emotional liability in subjects with high neuroticism.
Introduction
Personality traits describe recurrent patterns of thoughts, feelings, and actions that occur in response to situational demands [1]. The Big Five Model is perhaps the most influential model to describe human personality through five main personality traits: Extraversion, Neuroticism, Agreeableness, Conscientiousness, and Openness to experience [2]. According to Eysenck’s (1967) theory of personality, neuroticism is interlinked with low tolerance for stress or aversive stimuli. Those subjects scoring higher in neuroticism are emotionally more reactive and vulnerable to stress [3]. Furthermore, high neurotic individuals express heightened emotional reactivity, especially to negative events [4]. They tend also to be more self-critical [5] and overly sensitive to criticism made by others [6]. They are more likely to interpret ordinary situations as threatening, and minor frustrations as hopelessly difficult, and these might contribute to a predisposition toward mood and anxiety disorders and thus present a risk factor towards these diseases [7-9] . At the other end of the scale, individuals who score low in neuroticism are less easily upset and areless emotionally reactive. They tend to be calm, emotionally stable, and free from persistent negative feelings [10,11].
Since subjects with high neuroticism are at risk to develop depressive and anxiety disorders and show low tolerance for stress, it is important to understand the biology of neuroticism. Neuroimaging may provide some insights into the neurobiological underpinnings of neuroticism. Most neuroticism-related functional MRI (fMRI) differences have been found in task-based studies [12-16]. It has been suggested that deficiencies in functional circuits in neuroticism may be associated to topographic characteristics of resting state networks [17]. A growing interest in the use of resting state fMRI, which does not require a task, has risen over the last few years. Resting state fMRI allows for the examination of large scale neural systems that exhibit spontaneous synchronous fluctuations during non-goal directed fluctuations, such as wakeful rest, sleep and anaesthesia [18]. Correlations between these low frequency (less than 0.1Hz) spontaneous fluctuations on Blood Oxygenated Level Dependent (BOLD) signal are considered to reflect interactions between adjacent and non-adjacent brain areas that form spatially distributed networks of brain function [19]. Resting State Functional Connectivity (RSFC) is the observed correlation in spontaneous neural activity between brain areas at rest [17].
To improve understanding of RSFC in adults with high scores for neuroticism, the coordinates for regions of interest (ROIs) involved in neuroticism neuro pathophysiology [12] from five neural networks were extracted [20,21]. These coordinates were used as correlates with all other time series in the brain to determine temporally coherent networks of RSFC [21].
The first network included in our study was the affective network. This network contains integrated regions of the affective sub-division of the Anterior Cingulated Cortex (ACC), amygdale, nucleus accumbens, hypothalamus, anterior insular, hippo campus and orbit frontal cortex with reciprocal connections to autonomic, visceromotor and endocrine systems [21]. This network is involved in emotional regulation and monitoring of salience of motivational stimuli [21].A very recent fMRI resting state study showed significantly increased correlation in the bilateral amygdale in people with neuroticism [22]. Studies using cognitive-affective tasks found that activations in the frontal cortex, Dorsomedial Prefrontal Cortex (DMPFC), and amygdale are related to neuroticism [15,23,24] Moreover, higher levels of neuroticism have been associated with a stronger interaction between the right amygdale and the right hippocampus as well as the right amygdala and prefrontal cortical regions, specifically ventromedial prefrontal cortex, dorsolateral prefrontal cortex, and ACC, in an aversive pavlovian conditioning task [25]. Since affective disorders are highly associated with neuroticism [26,27] it is important to take studies in the area of affective disorders into account. Previous resting state studies examining affective disorders have found altered connectivity of the above described structures [28,29]. Heightened functional connectivity for high neuroticism participants compared to low neuroticism participants was expected in our study.
The second network, the ventral attention network, uses the Temporoparietal Junction (TPJ) and Ventral Frontal Cortex (VFC) to reorient attention to salient behaviourally relevant stimuli [20]. No study to date has yet investigated this network in people with high neuroticism scores. However, Sylvester and colleagues have recently shown that children with a history of depression and/or anxiety had reduced RSFC among the regions of the ventral attention network compared to children with no psychiatric history [30]. Thus, hypoconnectivity in this network is to be expected in people with high scores for neuroticism.
Our third network is the bilateral dorsal attention network. This network uses regions such as the Intraparietal Sulcus (IPS) and the Frontal Eye Field (FEF) to enable the control of spatial attention through selection of sensory stimuli based upon internal goals or expectations and links them to appropriate motor responses, a topdown orienting of attention [20]. There is no study in the research literature looking at this network and neuroticism or mood disorders but we found a recent study by Sripada looking at an emotional regulation strategy of reappraisal. The authors showed that people who engaged in the task of reappraisal had increased connectivity in the dorsal attention and default networks [31]. We also expect alterations in connectivity within this network for the group with high levels of neuroticism.
The fourth network we included in our study was the cognitive control network which utilizes regions such as the bilateral Dorsolateral Prefrontal Cortex (DLPFC), pre-supplementary motor area, inferior frontal junction, anterior insular cortex, dorsal-premotor cortex, and posterior cortices [32]. These regions have been implicated in behavioural inhibition, suppression of unwanted thoughts, attention shifting, and efforts to reappraise emotional stimuli [33-35]. Recent work using resting state fMRI suggests that depression is associated with abnormalities in the functional connectivity between these regions, which comprise key nodes of the so-called cognitive control network [21,36,39]. Clasen and colleagues have shown in a very recent study that alterations within the cognitive control network predated the onset of depression in young women with familiar risk for depression. Cognitive control network connectivity could then be considered a viable risk factor for depression [40]. As we mentioned earlier the task-based study by Tzschoppe has shown that higher levels of neuroticism are associated also with a stronger interaction between the right amygdala and prefrontal cortical regions, specifically Ventromedial Prefrontal Cortex (VMPFC), DLPFC and ACC [25]. High neuroticism poses a risk for depression so we hypothesized that these individuals should also show an altered connectivity in the cognitive control network.
The fifth network that we have considered in the study is the default network. It contains the precuneus/posterior cingulated cortex, the medial prefrontal cortex, dorsal ACC, and acts as a form of functional connectivity baseline thought to reflect intrinsic brain activity [17]. It has been documented in the literature that patients with depression show altered default mode network connectivity at several regions [41,42]. Kunisato and colleagues have observed that neuroticism correlated negatively with regional activity in the middle frontal gyrus and the precuneous (the latter being part of the default network model) [43,44,]. We expect to replicate this finding in our sample as there are no other studies on neuroticism and resting state fMRI.
The aim of our study is to explore the RSFC in individuals with high scores for neuroticism and to compare them with those scoring low neuroticism. We want to determine the localization and specificity of neuroticism-related connectivity differences between the two groups and to confirm our hypothesis that high neuroticism shows altered connectivity at several resting state network models that may resemble the changes found in depression (as neuroticism is a well-documented risk factor for depression).
Methods
Participants and rating scales
Forty five healthy participants were included in the study sample (28 females and 17 males). All participants were interviewed, and screened for any potential psychiatric diagnosis according to exclusion criteria and using the Structured Clinical Interview for DSM-IV (SCID-I) [45] by two psychiatrists. Exclusion criteria included: previous history of head injury or medical illness, previous history of mental illness, previous history of taking medication or substance/alcohol abuse. Handedness was obtained from the Edinburgh Handedness Inventory [46]. Self-administered and observer-rated scales were completed by all participants. The rating scales used were: the SCID-II personality questionnaire [45], the NEO Five Factor Inventory questionnaire [2], the Hamilton Rating Scale for Depression [47], the Hamilton Anxiety Inventory [47] and the Beck Depression Inventory [48]. The NEO Five Factor personality inventory is designed to assess the five factors or dimensions of personality: neuroticism, openness to experience, agreeableness, extraversion and conscientiousness. It includes 60 items, 12 for each dimension of personality. The cut off points for neuroticism are as follows: below 13 for low neuroticism in males (below 6 for very low), below 16 for low neuroticism in females (below 8 for very low), above 21 for high neuroticism in males (above 29 for very high) and above 25 for high neuroticism in females (above 32 for very high) [2].
Ethical approval for the study was granted by the Ethics Committee of Adelaide and Meath Hospital incorporating the National Children’s Hospital. Each participant gave written consent prior to participation in the study, and oral and written information about the project was given to all participants.
MRI methods
Magnetic resonance images from each participant were obtained with a Philips Achieve MRI scanner (Philips Medical System, Netherland BV, Veenphuis 4-6, 5684 PC Best, and The Netherlands) operating at 3 Tesla. The functional images were collected in single runs using a gradient echo (TE=28ms; TR=2000; field of view=131mm, flip angle=90°) sensitive to Blood Oxygenation Level-Dependent (BOLD) contrast (T2* weighting). A total of 37 contiguous 3.2 mm-thick slices were acquired parallel to the anterior posterior commissural plane (3mm approximately isotropic resolution), providing complete brain coverage. The fMRI run included 220 volumes acquired continuously lasting 7.2 min in total. Structural data (for definitive atlas transformation) included a high resolution sagittal, 3D T1-weighted Turbo Gradient Echo Sequence (TE=3.9ms, TR=8.5ms, TI=1060ms, flip angle=8°), 256×240 acquisition matrix, 1×1×1 mm voxels) scan.
Pre-processing of functional data
Using SPM8 (https://www.fif.ion.ucl.ac.uk/spm/software/spm8) fMRI data were pre-processed using the following steps: first, compensation of systematic, slice-dependent time shifts; second, the elimination of systematic odd-even slice intensity differences due to interleaved acquisition; and third, rigid body correction for inter frame head motion within and across runs. Data were excluded if motion parameters exceeded 3 mm in any direction or 3.0° of any angular motion throughout the course of the scan. Next, coregistration of the structural T1 image to the functional scans was carried out. Spatial normalization to standard 3mm×3mm×3mm Montreal Neurological Institute space was then applied to the functional images and to the structural image respectively to allow for inter-subject analysis. Functional resting state data were then spatially smoothed (smoothing full width at half maximum=8mm).
Using CONN resting state software (https://www.nitrc.org/ project/conn/) the data were temporally band-pass filtered (.009<f<0.08). Several sources of spurious variance along with their temporal derivatives then were removed from the data by linear regression, such as signal from regions cantered in the white matter, cerebrospinal fluid and movement. CONN implements the component-based noise correction method (CompCor) strategy for physiological and other noise source reduction [49]. CompCor has the advantage of not requiring external monitoring of physiological fluctuations. Compared to methods that rely on global signal regression, the CompCor noise reduction method allows for interpretation of anti correlations as there is no regression of the global signal. This approach may enhance the sensitivity of positive correlations and produce comparable negative correlations [50]. This regression procedure removes fluctuations unlikely to be involved in specific regional correlations.
Functional connectivity analysis of resting state activity
To compute functional connectivity maps corresponding to a selected seed Region of Interest (ROI), the region time course was correlated against all other voxels within the brain. Based on data from two previous resting state studies [20,21] connectivity withinthe affective network, ventral attention system, dorsal attention system, cognitive control network and default mode network were explored. Correlation maps were produced by extracting the BOLD time course from a seed region, then computing the correlation coefficient between that time course and the time course from all other brain voxels. The following seed ROIs with 5 mm radius were extracted for these 5 specific neural networks: the ACC (+/- x=10, y=- 35, z=2) in the affective network; the TPJ (+/- x=53, y=-48, z=20) and VFC (+/- x=37, y=-18, z=1) in the ventral attention system; the IPS (+/- x=-27, y=-58, z=49) and FEF (+/- x=24, y=-13, z=51)in the dorsal attention system, the DLPFC (+/- x=36, y=27, z=29) in the cognitive control network and the precuneus (+/- x=7, y=-60, z=21) in the default mode network. For the ventral (TPJ, VFC) and dorsal (IPC, FEF) attention network we thus extracted 2 connectivity maps. The principal techniques used were computation of whole brain voxelwise intrinsic functional connectivity maps.
Statistical analysis
We analysed resting state functional MRI data in SPM8 using two-sample t-test to determine significant differences in functional connectivity between high and low neuroticism using the cut off points described above, and using a Family Wise Error (FWE) whole brain corrected threshold of P<0.01 (FWE corrected for the whole brain). The threshold was reduced to P<0.01 from P<0.05 because we analysed connectivity within the 5 different networks. Mean connectivity data were extracted from areas that showed significant differences between high and low neuroticism participants. Since Beck’s Depression Inventory and Hamilton Anxiety and Depression Rating Scales scores were different between participants with low and high neuroticism (but still all below the threshold for depression or anxiety disorder) these were added as covariates in additional ANCOVA analyses in SPM to observe the difference between participants with high neuroticism scores and participants with low neuroticism scores. Moreover, to assess for linear effects and associations between neuroticism as well as age and depression/ anxiety scores and brain connectivity we used Spearman’ rank test in regions that showed significant differences between individuals from the high neuroticism group versus those from the low neuroticism group in an additional analysis.
Results
Twenty eight female participants and 17 male participants entered the study. Ten participants scored high levels of neuroticism on the NEO Five Factor personality inventory. Compared to low neuroticism group, participants with high neuroticism scores displayed significantly greater functional connectivity in the right affective network (Table 1). We found significant differences at the right ACC. Participants with high neuroticism scores showed significantly greater functional connectivity between the right ACC and the left superior parietal lobe (x=-46, y=-56, z=56) as well as the right superior medial frontal lobe (x=-4, y=30, z=44) and the right inferior parietal lobe (x=54, y=-58, z=48) (Figure 1).
Networks and regions
FWE-Corr
K value
t
x
y
z
Affective network
R ACC-left sup parietal lobe
0.025
159
0.001
-46
-56
56
R ACC-right inferior parietal lobe
0.020
159
0.001
54
-58
48
R ACC-right sup medial frontal lobe
0.000
321
0.000
-4
30
44
Ventral attention network
…
Dorsal attention network
…
Cognitive control network
…
Default mode network
…
Table 1: Between group resting state connectivity differences: high neuroticism >low neuroticism.
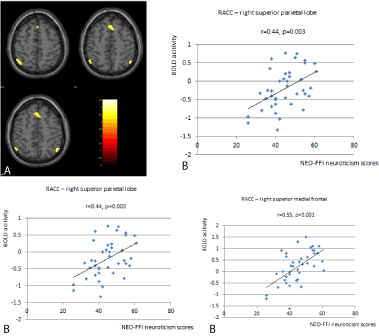
Figure 1: Participants with high neuroticism scores showed significantly
greater functional connectivity between the right ACC and the right inferior
parietal lobe (x=54, y=-58, z=48) in comparison to participants with low
neuroticism scores (1A). The individual BOLD activity is depicted in relation
to neuroticism scores on the right side of the figure (1B).
There was significantly less functional connectivity in the cognitive control network for participants with high neuroticism scores when compared to those with low neuroticism scores (Table 2). We found significant differences between the right DLPFC with the right middle temporal lobe (x=60, y=-34, z=2) and the right insula (x=36, y=-16, z=16) (Figure 2). There was also less functional connectivity in the ventral attention network for participants with high neuroticism scores when compared to those with low neuroticism scores (Table 2). Here significant differences between the right TPJ and the left inferior parietal lobe were detected (x=-46, y=-52, z=40) (Figure 3).
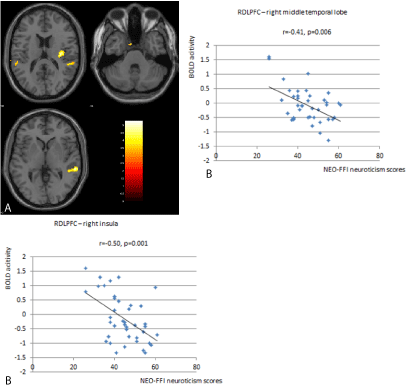
Figure 2: Significant differences between the right DLPFC with the right
middle temporal lobe (x=60, y=-34, z=2) and the right insula (x=36, y=-16,
z=16) were found in the study. Participants with high neuroticism show
significantly lower RSFC in these areas compared to participants with lower
neuroticism (2A). The individual BOLD activity is depicted in relation to
neuroticism scores on the right side of the figure (2B).
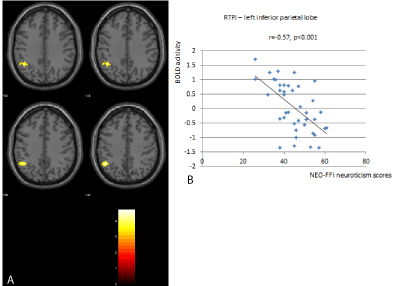
Figure 3: There were significant differences between the right TPJ and the left
inferior parietal lobe (x=-46, y=-52, z=40). Participants with high neuroticism
show significantly lower RSFC in these areas compared to participants with
lower neuroticism (3A). The individual BOLD activity is depicted in relation to
neuroticism scores on the right side of the figure (3B).
Networks and regions
FWE-Corr
K value
T
x
y
z
Affective network
…
Ventral attention network
R TPJ-left inferior parietal lobe
0.027
160
0.001
-46
-52
40
Dorsal attention network
…
Cognitive control network
R DLPFC-right mid temporal lobe
0.004
235
0.000
60
-34
2
R DLPFC-right insula
0.025
163
0.001
36
-16
16
Default mode network
…
Table 2: Between group resting state connectivity differences: low neuroticism >high neuroticism.
We should also mention that all group comparison results held true when Beck’s Depression Inventory and Hamilton Anxiety and Depression Rating Scales scores were included as co-varieties in the analysis.
No significant correlations were found between RSFC and age, depression or anxiety scores (Table 3).
Age
NEO
FFI
HAM_D
HAM_A
BDI
RACC_ left sup parietal lobe
Corr_coef
.061
.443
.086.
.289
.186
Sig.
.696
.003
.584
.064
.231
RACC_ right inferior parietal lobe
Corr_coef
-.096
.461
-.061
.230
.169
Sig.
.542
.002
.697
.142
.278
RACC_RDMPFC
Corr_coef
.163
.552
-.052
.295
.223
Sig.
.297
.000
.739
.058
.155
RDLPFC_ right mid temporal lobe
Corr_coef
-.179
-.412
-.093
-.166
.000
Sig.
.252
.006
.554
.293
1.00
RDLPFC_right_insula
Corr_coef
.249
-.501
-.197
-.358
-.351
Sig.
.107
.001
.205
.020
.021
RTPJ_ left inferior parietal lobe
Corr_coef
.200
-.567
.113
-.150
-.127
Sig.
.199
.000
.471
.342
.418
Table 3: There were significant differences between the right TPJ and the left inferior parietal lobe (x=-46, y=-52, z=40). Participants with high neuroticism show significantly lower RSFC in these areas compared to participants with lower neuroticism (3A). The individual BOLD activity is depicted in relation to neuroticism scores on the right side of the figure (3B).
Discussion
To the best of our knowledge, this is the first time the affective, cognitive control and attention networks were explored together with the default mode network to determine the localization and specificity of neuroticism-related RSFC differences in adults with high and low neuroticism scores.
A main finding within our study was as hypothesized the significantly higher RSFC within the affective network for participants with high neuroticism scores when compared to those with low neuroticism scores. As we said earlier, this network is involved in emotional regulation and monitoring the salience of motivational stimuli [21].Our finding is in line with findings that subjects with high neuroticism are more emotional reactive and stress sensitive. We found that participants with high neuroticism displayed more RSFC between the right ACC of the affective network and the left superior parietal lobe, the right inferior parietal lobe and the right superior medial frontal lobe (DMPFC).
The ACC has been very much associated with emotional regulation [51-54], the experience of sadness and anxiety [55,56] and with depression [57]. The dorsal part of the ACC is connected with the prefrontal and parietal cortices as well as the motor system and the frontal eye fields making it a central station for processing top-down and bottom-up stimuli and assigning appropriate control to other areas in the brain [58]. Thus, our findings that functional connectivity between ACC and prefrontal and parietal cortices is relevant for neuroticism is in line with these anatomical connections. Interestingly, resting state studies examining affective disorders have also found altered connectivity of the affective network, in particular in the ACC and DMPFC [28,29]. FMRI BOLD responses in the frontal cortex, DMPFC and amygdala have also been associated with neuroticism in cognitive-affective task studies [12,15,24,59,60] ft superior parietal lobe is known to be involved in body part localization [61], writing [62] and manipulation of information in working memory [63]. The right superior medial frontal lobe is involved in theory of mind processes [64] and inhibitory control [65]. These parietal and frontal areas of the brain have been closely related to anxiety and mood disorders [66,67] and high neuroticism seems to predispose to mood and anxiety disorders [7,8,9].
The cognitive control network showed also interesting and novel results. The brain areas included in this network have been implicated in behavioural inhibition, suppression of unwanted thoughts, attention shifting, and efforts to reappraise emotional stimuli [33-35]. Participants with high neuroticism scores exhibited significantly less connectivity between the DLPFC and the right middle temporal lobe and the right insula.
The DLPFC has been linked with adaptive control [32] working memory [68] and emotional regulation [69]. The middle temporal lobe was found to be less connected with the DLPFC in our study. Previous neuroimaging studies have suggested that the middle temporal lobe is associated with language (Brodmann area 21) and semantic memory processing [70-72]. A study by Kennedy et al., 2007 using PET scans found that depressed patients treated with venlafaxine showed increased metabolism in the Brodmann 21 area. An opposite pattern was found for patients treated with cognitive behavioural therapy [73].
The insulin was also significantly disconnected to the DLPFC in individuals with high neuroticism scores. The insulin is instrumental in integrating disparate functional system involved in processing affect, sensory-motor processing, and general cognition and is well suited to provide an interface between feelings, cognition and action [74]. This hypo connectivity is relevant as other fMRI resting state studies have shown hypo activation of cognitive control network predating the onset of depression in young women with familiar risk for depression. Cognitive control network connectivity could be then considered a potential risk factor for depression [40].
The ventral attention network reorients attention to salient behaviourally relevant stimuli [20]. We found that individuals with high neuroticism scores showed less RSFC between the right TPJ and the left inferior parietal lobe. The right TPJ is pivotal in analysing signals from self-produced actions as well as from external environment [75]. The right TPJ is involved also in the identification and processing of social cues [76]. The inferior parietal lobe seems to be associated with detection of salient new events in the environment [77,78] and with sustained attention on task goals [79-81]. Problems with attention focus and distractibility are some of the cores symptoms of major depression and anxiety [45]. Sylvester and colleagues have recently showed that children with a history of depression and/or anxiety had reduced RSFC among the regions of the ventral attention network compared to children with no psychiatric history [30].
Our findings must be considered in light of limitations. The rating scale that we use to assess level of neuroticism is a self-rated one which despite of being reliable and validated tool is not free from recall bias. The cross-sectional design did not involve follow-up with research participants. Therefore, we were not able to determine if differences in connectivity predated depression or anxiety disorder onset, reflecting neuroticism as a risk factor for development of mood/ anxiety disorders. Including longitudinal follow up is a critical feature of future research to directly assess the extent to which differences in connectivity predict depression onset, particularly in the context of life stress. The sample size was large enough to look at differences between participants with high and low neuroticism scores. We chose this procedure, since neuroticism might not be a linear function of brain function that would be the requirement for a linear regression analysis. Strength of our study is that our results are valuable as they were corrected for multiple comparisons and strong enough to survive conservative Bonferroni testing, which reduced type 1 errors.
Conclusion
In conclusion, our findings shed light upon the neuroticism related RSFC and its role as a risk factor for the development of major depression. High neuroticism was associated with affective network hyper connectivity and cognitive control and ventral attention network hypo connectivity. Affective network hyper connectivity might be related to emotional problems or mood disorders that are associated with high neuroticism. Additionally, the hypo connectivity seen in the cognitive control might have to do with the inattention and cognitive deficits that have consistently been found in major depression and anxiety disorders. Thus, oversensitivity in affective systems and at the same time reduced cognitive control might be in line with increased stress sensitivity and emotional liability in subjects with high neuroticism.
References
- Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004; 55: 1-22.
- Costa JF, Mccrae RR. Revised NEO Personality Inventory (NEO-PI) and NEO Five-Factor Inventory (NEO-FFI), Odessa, FL. 1992.
- Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007; 44: 823-826.
- Canli T. Toward a neurogenetic theory of neuroticism. Ann N Y Acad Sci. 2008; 1129: 153-174.
- Clara IP, Cox JB, Enns MW. Hierarchical models of personality and psychopathology: the case of self-criticism, neuroticism, and depression. Pers Individ Dif. 2003; 35: 91-99.
- Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. J Abnorm Psychol. 1994; 103: 18-31.
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005; 186: 190-196.
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001; 158: 1673-1679.
- Lahey BB. Public health significance of neuroticism. Am Psychol. 2009; 64: 241-256.
- Lucas RE, Fujita F. Factors influencing the relation between extraversion and pleasant affect. J Pers Soc Psychol. 2000; 79: 1039-1056.
- Lucas RE, Diener E, Grob A, Suh EM, Shao L. Cross-cultural evidence for the fundamental features of extraversion. J Pers Soc Psychol. 2000; 79: 452-468.
- Servaas MN, Van Der Velde J, Costafreda SG, Horton P, Ormel J, Riese H, et al. Neuroticism and the brain: a quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neurosci Biobehav Rev. 2013; 37: 1518-1529.
- Servaas MN, Riese H, Renken RJ, Marsman JB, Lambregs J, Ormel J, et al. The effect of criticism on functional brain connectivity and associations with neuroticism. PLoS One. 2013; 8: e69606.
- Di Simplicio M, Norbury R, Reinecke A, Harmer CJ. Paradoxical effects of short-term antidepressant treatment in fMRI emotional processing models in volunteers with high neuroticism. Psychol Med. 2014; 44: 241-252.
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci. 2001; 115: 33-42.
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci. 2005; 5: 169-181.
- Deco G, Corbetta M. The dynamical balance of the brain at rest. Neuroscientist. 2011; 17: 107-123.
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012; 16: 17-26.
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98: 676-682.
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006; 103: 10046-10051.
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010; 107: 11020-11025.
- Gao Q, Xu Q, Duan X, Liao W, Ding J, Zhang Z, et al. Extraversion and neuroticism relate to topological properties of resting-state brain networks. Front Hum Neurosci. 2013; 7: 257.
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007; 121: 249-256.
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cogn Affect Behav Neurosci. 2009; 9: 1-15.
- Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, et al. Aversive Learning in Adolescents: Modulation by Amygdala-Prefrontal and Amygdala-Hippocampal Connectivity and Neuroticism. Neuropsychopharmacology. 2013.
- Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med. 2007; 37: 453-462.
- Jylha P, Melartin T, Isometsa E. Relationships of neuroticism and extraversion with axis I and II comorbidity among patients with DSM-IV major depressive disorder. J Affect Disord. 2009; 114: 110-121.
- Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013; 150: 727-735.
- Tahmasian M, Knight DC, Manoliu A, Schwerthoffer D, Scherr M, Meng C, et al. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front Hum Neurosci. 2013; 7: 639.
- Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J Am Acad Child Adolesc Psychiatry. 2013; 52: 1326-1336.
- Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, et al. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2013.
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007; 37: 343-360.
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004; 8: 170-177.
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006; 16: 1679-1689.
- Hampshire A, Thompson R, Duncan J, Owen AM. Lateral prefrontal cortex subregions make dissociable contributions during fluid reasoning. Cereb Cortex. 2011; 21: 1-10.
- Schlosser RG, Koch K, Wagner G, Nenadic I, Roebel M, Schachtzabel C, et al. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008; 46: 336-347.
- Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009; 39: 977-987.
- Veer IM, Beckmann CF, Van Tol MJ, Ferrarini L, Milles J, Veltman DJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010; 4.
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012; 139: 56-65.
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014; 7: 13-22.
- Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med. 2013.
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013; 74: 898-907.
- Kunisato Y, Okamoto Y, Okada G, Aoyama S, Demoto Y, Munakata A, et al. Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci Res. 2011; 69: 129-134.
- Kunisato Y, Okamoto Y, Okada G, Aoyama S, Nishiyama Y, Onoda K, et al. Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neurosci Lett. 2011; 492: 109-113.
- First MB SR WJ. Structured clinical interview for DSM-IV axis II disorders SCID-II: clinician version, administration booklet. American Psychiatric Pub. 1997.
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9: 97-113.
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959; 32: 50-55.
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988; 8: 77-100.
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007; 37: 90-101.
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012; 59: 1420-1428.
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005; 45: 651-660.
- Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005; 17: 874-883.
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004; 16: 1746-1772.
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004; 23: 483-499.
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003; 3: 207-233.
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003; 126: 2139-2152.
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011; 69: 301-308.
- Posner, Michel, Gregory J. "Executive attention: Conflict, target detection, and cognitive control". The attentive brain, Cambridge, Mass, MIT Press. 1998.
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007; 27: 6313-6319.
- Kehoe EG, Toomey JM, Balsters JH, Bokde AL. Personality modulates the effects of emotional arousal and valence on brain activation. Soc Cogn Affect Neurosci. 2012; 7: 858-870.
- Felician O, RomaiguÉre P, Anton JL, Nazarian B, Roth M, Poncet M, et al. The role of human left superior parietal lobule in body part localization. Ann Neurol. 2004; 55: 749-751.
- Menon V, Desmond JE. Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Brain Res Cogn Brain Res. 2001; 12: 337-340.
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009; 29: 14980-14986.
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on 'Theory of Mind' and cognition. Brain. 2004; 127: 914-928.
- Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006; 18: 1843-1849.
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007; 27: 6313-6319.
- Willeumier K, Taylor DV, Amen DG. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Transl Psychiatry. 2011; 1: e28.
- Mars RB, Grol MJ. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J Neurosci. 2007; 27: 1801-1802.
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012; 7: e48107.
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000; 12: 1-47.
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997; 35: 1319-1327.
- Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J Cogn Neurosci. 2012; 24: 1766-1778.
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007; 164: 778-788.
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013; 23: 739-749.
- Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends Cogn Sci. 2002; 6: 237-242.
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007; 13: 580-593.
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000; 123: 1624-1633.
- Huang MX, Lee RR, Miller GA, Thoma RJ, Hanlon FM, Paulson KM, et al. A parietal-frontal network studied by somatosensory oddball MEG responses, and its cross-modal consistency. Neuroimage. 2005; 28: 99-114.
- Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001; 42: 266-272.
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. Functional specificity of superior parietal mediation of spatial shifting. Neuroimage. 2001; 14: 661-673.
- Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, Kemna S, et al. Network for auditory intrinsic alertness: a PET study. Neuropsychologia. 2004; 42: 563-568.