
Research Article
Ann Depress Anxiety. 2014;1(7): 1032.
Antidepressant and Anxiolytic Potentials of Chebulinic Acid in Laboratory Rodent
Onasanwo SA1,2*, Faborode SO1, Agrawal M2, Ijiwola OL1, Jaiyesimi BO1 and Narender T2
1Department of Physiology, University of Ibadan, Nigeria
2Medicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, India
*Corresponding author: Onasanwo SA, Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Ibadan, Nigeria
Received: October 15, 2014; Accepted: November 17, 2014; Published: November 18, 2014
Abstract
Chebulinic acid, an ellagitannin, present in the fruits of Terminalia chebula has shown various positive neurological properties. However, there has not been a report of its antidepressant and anxiolytic potentials. This study was conducted to investigate the antidepressant and anxiolytic potentials of chebulinic acid in laboratory rodents.
Chebulinic acid was evaluated for its antidepressant (Forced swimming test and Tail suspension test) and anxiolytic (Light-dark box test, Hole-board test and Elevated plus maze) potentials in mice.
Our findings showed antidepressant-like activity which is dose consistent at p<0.01 (in forced swimming test) and p<0.05 (in tail suspension test). However, only doses 20mg/kg and 40mg/kg showed significant anxiolytic potentials in elevated plus maze, light-dark box test and hole-board paradigms when compared with the control (P<0.05). The results suggest antidepressant and anxiolytic potentials of chebulinic acid.
Chebulinic acid may possess antidepressant and anxiolytic potentials which may correspond to its folkloric use in the treatment of neuro-physiological disorders.
Keywords: Anti-depressant; Anxiolytic; Chebulinic acid; Forced swimming test; Tail suspension test
Introduction
Depression is a common mental disorder that presents with depressed mood, loss of interest or pleasure, decreased energy, feelings of guilt or low self-worth, disturbed sleep or appetite, and poor concentration. Moreover, depression often comes with symptoms of anxiety. Depression is a significant contributor to the global burden of disease and affects people in all communities across the world. Today, depression is estimated to affect 350 million people. The World Mental Health Survey conducted in 17 countries found that on the average, about 1 in 20 people reported having an episode of depression in the previous year. At its worst, depression can lead to suicide. Almost 1 million lives are lost yearly due to suicide, which translates to 3,000 suicide deaths every day. For every person who completes a suicide, 20 or more may attempt to end his or her life. The demand for curbing depression and other mental health conditions is on the rise globally. A recent World Health Assembly called on the World Health Organization and its member states to take action in this direction [1].
Anxiety and Depression are major psychiatric disorders, which affect the daily lives of individuals experiencing them. Currently, there are many marketed drugs that have been used in the treatment of the most prevalent psycho-pathological conditions commonly found in the Western World, including depression and anxiety among other conditions. Many of these marketed drugs have one or more negative side effects or the other. Based on these facts, the employment of natural products with infinitesimally, noticeable side-effects constitute substitutes for treating these neuro- and psychopathologies.
The plant Terminalia chebula also known as Haritaki has an esteemed origin in Indian mythology. Its fruits have been used to treat many diseases such as digestive problems, diabetes, colic pain, chronic cough, sore throat, asthma, bleeding piles, vomiting, gout, e.t.c. Terminalia chebula is a deciduous tree growing up to 30-metre (98 ft) tall, with a trunk up to 1-metre (3 ft 3 in) in diameter. The fruits are drupe-like, long, broad and blackish, with five longitudinal ridges and are hard and yellowish-green in colour. Chebulinic acid is an ellagitannin found in the fruits of Terminalia chebula. It has the molecular formular of C41H32O27 and molecular weight of 956.67658 [g/mol]. It is isolated from the fruits of Terminalia chebula by highspeed counter-current chromatography [2]. Other studies have shown that chebulinic acid has therapeutic activities which include antihypertensive activity [3] and antiulcerogenic activity [4].
However, there has not been any report of the antidepressant and anxiolytic activities of chebulinic acid. Therefore, the objective of this present study is to evaluate the antidepressant and anxiolytic-like potentials of chebulinic acid in laboratory mice using depression and anxiety models.
Materials and Methods
Plant material and extraction procedure
The dried fruits of Terminalia chebula were purchased from the local market in Lucknow, India and the authentication was done by the botany division of Central Drug Research Institute, Lucknow, India (Chem. Reg. No. 109).
Dried fruits of Terminalia chebula after removing the seeds were hammered into small pieces and were placed in glass percolator with 5L ethanol: distilled water (1:1) and allowed to stand at room temperature for 24hours. The percolate was collected and this process was repeated four times. The combined percolate was concentrated under vacuum using rotary evaporator at 40°C and weighed. The weight of extract was found to be 430gm.
The dark brown crude extract was macerated with hexane and was slowly decanted and this process was repeated three times and resulting hexane soluble part on concentrating under reduced pressure, gave the hexane fraction in negligible yield. The residue was then dissolved in water followed by fractionation with chloroform for three times. Resulting chloroform soluble part after removing solvent under reduced pressure at 40°C gave chloroform fraction with yield of 5g. Butanol fraction was obtained by fractionating the aqueous part by using n-butanol with the yield of 210g. The water soluble part was finally evaporated to give water fraction with yield of 90g.
Size exclusion column chromatography of the n-butanol fraction has been done over sephadex LH 20 using triple distilled water and methanol in varying polarity as mobile phase. Chebulinic acid was isolated from the 40% methanol water fractions with little impurities visible in reverse TLC which was then recrystalized using acetonitrite water (20:80) to afford a pure compound (8g). The compound gave the phenolic test with ferric chloride solution. Structure elucidation was performed by spectroscopic technique [4]. On the basis these data, this compound was identified as chebulinic acid which was confirmed by comparison of its physiochemical data with that reported in the literature [5].
Experimental animals
Adult male Swiss albino mice (20-25 g) were purchase and housed in raised bottom mesh cages to prevent coprophagy, in a well ventilated animal house, Department of Physiology, University of Ibadan, Ibadan, Nigeria, and were kept in environmentally controlled rooms (25 ± 2 °C, 12 hr light and dark cycles). Each mouse was used at once. They were fed with standard rat feed (Ladokun Feeds, Ibadan, Nigeria) and clean water ad libitum. All procedures in this study conformed to the guiding principles for research involving animals as recommended by the Declaration of Helsinki and the Guiding principles in the care and use of animals [6], and as approved by the Research Ethical Committee, University of Ibadan, Nigeria.
Depression models
Antidepressant activity of chebulinic acid was studied using two experimental procedures. These include the Forced Swimming Test and Tail Suspension Test. Doses of 10mg/kg, 20mg/kg and 40mg/kg of chebulinic acid were used. Imipramine (60mg/kg) was used as the reference anti-depressant drug. Test was carried out one hour after respective treatment of mice.
Forced swimming test: The Forced Swimming Test (FST) was performed according to the method of Porsolt, et al [7]. The apparatus consists of a clear plexiglass cylinder (20cm high by 12cm diameter) filled to a 15cm depth with water. Water to be used during this experiment is kept at a temperature (34 ± 1°C). Animals were divided into five groups. Each one of the animals was made to swim individually for fifteen minutes 24hrs prior to the test day. On the test day, the animals after 1hour of drug administration were made to swim for 6 minutes in which period of immobility were measured using a stopwatch. All drugs were administered orally. The period of immobility was not recorded during the first 1 minute. The animal was assumed to be immobile when it stopped escape movements and makes only movements necessary to keep its head above water.
Tail suspension test: The Tail Suspension Test (TST) was performed according to the method described by Steru, et al [8]. The mice were individually suspended 60cm above the surface of a table with an adhesive tape placed 1cm away from the tip of the animal’s tail. Immobility duration was recorded for the last 5 minutes during the 6-minute test. Mice were considered immobile only when they hung passively and were completely motionless. Imipramine (60mg/ kg), chebulinic acid (10mg/kg, 20mg/kg, 40mg/kg) and vehicle were administered 1 hour before the test. All drugs were administered orally.
Anxiety models
Anxiety was studied using three experimental procedures. Each test procedure was made up of 5 groups of 6 mice each. Group 1 served as control. Group 2 was the positive control and received 1.5 mg/kg b.w. diazepam (standard drug). Groups 3, 4, and 5 received chebulinic acid (10mg/kg, 20mg/kg, 40mg/kg, b.w. respectively). Test was carried out 30 minutes after respective treatment of mice.
Light-dark test: This test was carried out as described by Shimada and co-workers [9] based on the innate aversion of rodents for brightly illuminated areas. The apparatus consists of a Plexiglas box with two compartments (20 cm × 20 cm), one of which was illuminated with white light while the other was dark. After respective treatment, each animal was placed individually at the junction of the light-dark compartment, facing the illuminated compartment. Recordings were made over a 5 minute-period, counting the time spent in the illuminated area, as well as the number of entries into each space [10].
Hole-board test: This test was conducted as described by Boissier & Simon [11]. The hole-board apparatus consists of a gray wooden box (40x40 cm, 2.2 cm thick) with 16 equidistant holes 3 cm in diameter in the floor. Animals were kept in a quiet laboratory before this experiment, at least, one hour prior to testing. Each animal was placed singly in the centre of the board opposite the observer and the number of head dipping into the hole was recorded over a 5-minute exploration period on the board. Head dipping was recorded only when both eyes disappeared into the hole [12].
Elevated plus maze: The standard elevated plus maze test described by Montgomery [13] is used to assess anxiety-like behavior in laboratory animals. In brief, the apparatus was composed of two open and two closed arms that radiated from a central platform to form a plus sign that is elevated to a height of 40 cm above the floor level. Thirty minutes after respective treatment; each experimental animal was placed on the centre of the platform, facing an open arm. The number of entries and the time spent in the open and closed arms were recorded during a 5 minute test period. The percentage of open arm entries was calculated for each animal. The apparatus was cleaned thoroughly between trials with damp and dry towels to remove any residue or odour [14].
Statistical Analysis
Results were expressed as mean ± S.E.M. Variance was analyzed using One-way Analysis Of Variance (ANOVA), followed by Newman – Keul’s multiple comparisons test. P < 0.05 was considered to be statistically significant.
Results and Discussion
Antidepressant-like activity of chebulinic acid in the FST and TST
In the FST (Figure 1), chebulinic acid at doses 10, 20 and 40mg/ kg, and imipramine (60mg/kg) reduced immobility time significantly compared to the control [F(4,25)=157.7; P<0.001] .
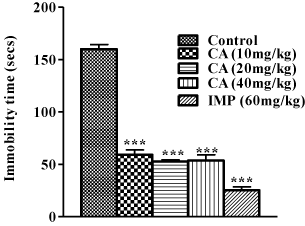
Figure 1: Effect of Chebulinic Acid (CA) and Imipramine (IMP) in mice
exposed to Forced Swimming Test (FST). Data represent means ± S.E.M of
6 mice during the 5 minute test session. Comparisons were made using oneway
ANOVA followed by post hoc Newman-Keul’s test. Statistical significant
at ***p < 0.001, in comparison to control.
In the TST (Figure 2), chebulinic acid at all doses used (10, 20 and 40mg/kg) showed a significance reduction of immobility time compared to the control [F(4,25)=51.1; P<0.05]. Also, oral administration of imipramine (60mg/kg) showed a significant reduction of immobility time compared to the control [F(4,25)=44.8; P<0.001].

Figure 2: Effect of Chebulinic Acid (CA) and Imipramine (IMP) in mice
exposed to Tail Suspension Test (TST). Data represent means ± S.E.M of 6
mice during the 5 minute test session. Comparisons were made using oneway
ANOVA followed by post hoc Newman-Keul’s test. Statistical significant
at *p < 0.05, ***p < 0.001, in comparison to control.
Anxiolytic activities of chebulinic acid in and elevated plus maze, light-dark, and hole board models
In the elevated plus maze test, neither pre-treatment with graded doses of chebulinic acid nor diazepam significantly increased the number of open arm entry when compared with the control that received vehicle as shown in Figure 3. However, there were significant increases in the time spent in open arms with pre-treatment with diazepam (0.5mg/kg), 20mg/kg and 40mg/kg of chebulinic acid as shown in Figure 4. Chebulinic acid at dose 10mg/kg could not increase amount of time spent in the open arm when compared to the control [F(4,25)=22.91; P<0.001).
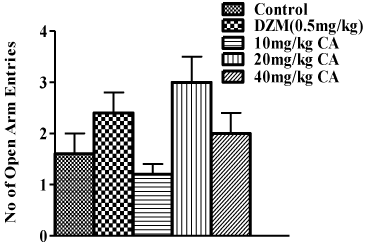
Figure 3: Effect of Chebulinic Acid (CA) and Diazepam (DZM) on number
of open arm entries in mice exposed to Elevated plus Maze (EPM). Data
represent means ± S.E.M of 6 mice during the 5 minute test session.
Comparisons were made using one-way ANOVA followed by post hoc
Newman-Keul’s test.
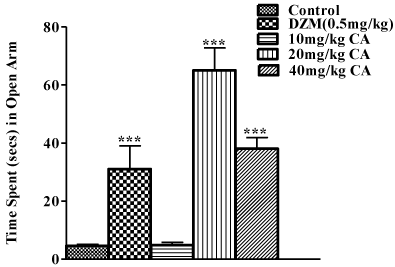
Figure 4: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
In the light and dark box test, pre-treatment with diazepam (0.5mg/kg), 20mg/kg and 40mg/kg of chebulinic acid significantly increased time spent in light box when compared with the control [F(4,25)=41.94; P<0.05]. Chebulinic acid at the dose of 10mg/kg could not reverse the reduced time spent in the light box when compared with the control as shown in Figure 5.
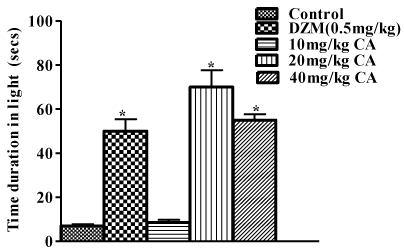
Figure 5: Effect of Chebulinic Acid (CA) and Diazepam (DZM) on time spent
in light box in mice exposed to light and dark box. Data represent means ±
S.E.M of 6 mice during the 5 minute test session. Comparisons were made
using one-way ANOVA followed by post hoc Newman-Keul’s test. Statistical
significant at *p < 0.05 in comparison to control.
In the hole board test, pre-treatment with diazepam (0.5mg/ kg) significantly increased (p<0.05) number of head dipping when compared to the control. Also, pre-treatment with chebulinic acid at doses of 20mg/kg and 40mg/kg also increased the number of head dippings [F(4,25)=78.28; P<0.05]. When compared to the vehicle treated group. However, chebulinic acid at a dose of 10mg/kg could not increase the number of head dipping when compared to the control as shown in Figure 6.
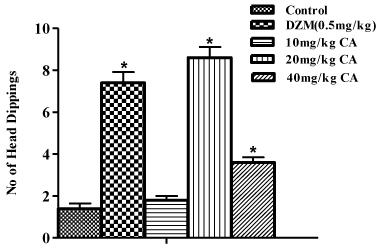
Figure 6: Effect of Chebulinic Acid (CA) and Diazepam (DZM) on no of
head dippings in mice exposed to whole board test. Data represent means ±
S.E.M of 6 mice during the 5 minute test session. Comparisons were made
using one-way ANOVA followed by post hoc Newman-Keul’s test. Statistical
significant at *p < 0.05 in comparison to control.
In the present study we showed, to our knowledge for the first time, that chebulinic acid isolated Terminalia chebula produces significant antidepressant-like and anxiolytic effects when assessed in Forced Swimming Test (FST) and Tail Suspension Test (TST), Elevated Plus Maze (EPM), light-dark box and hole board paradigms.
Our results show that chebulinic acid displayed behavioral profile that is consistent with an antidepressant and anxiolytic actions. The forced swimming test and tail suspension test are the widely used pharmacological in-vivo model for assessing antidepressant and anxiolytic activities [15,16]. Chebulinic acid caused a significant reduction in the duration of immobility using this test, suggesting an antidepressant-like effect. Simple procedures (light-dark, hole-board, and elevated plus maze) for examining response of an animal to unfamiliar environment were used to assess anxiety and/or responses of animals to stress [10]. The results showed that chebulinic acid possesses anxiolytic-like effects, and this is consistent in all paradigms.
The present data demonstrate that chebulinic acid has a clear and consistent effect on the time spent in illuminated areas in the lightdark test. In the hole-board test, the number of head-dips by the mice administered with 20mg/kg and 40mg/kg is comparable with results from diazepam treated group. The plus maze task approaches the conflict between the innate fear that rodent has for open areas versus their desire to explore new environments [17]. When anxious, the natural tendency of rodents is to prefer enclosed dark spaces to opened brightly lit spaces. In this context, anxiolytic effects of drugs in rodent are measured by the number of entries into and time spent in the open arms of the maze. A mouse was considered to have entered an arm when all four paws were on the arm [10]. In addition, increased number of entries and exploratory time in the open arm of the elevated plus maze paradigms further indicate an efficacy of chebulinic acid in the treatment of anxiety and stress related disorders. Exposure of the animals to novel maze-alley evokes an approach avoidance conflict which is stronger in open arm compared with closed arm. The plasma cortisol level has been reported to be increased in the open arms, a true reflection of anxiety [18]. When animals enter open arm, they freeze, become immobile, defecate and show fear-like movements. An increase in open arm activity (duration and number entries) after chebulinic acid treatment reflects anti-anxiety behavior [14].
Anxiolytics (e.g. diazepam) are known to exert their pharmacological action by increasing the gamma aminobutyric acid (GABA) content of mice cerebral hemisphere [12,19]. Further neuropharmacological studies will be required to ascertain if chebulinic acid actually mediates its action via similar mechanism.
Conclusion
This study reveals that chebulinic acid possesses anti-depressant and anxiolytic potentials. However, more machanistic studies are needed to further confirm our findings. This will be to investigate the involvement of cerebral monoaminergic transmitters such as norephinephrine, 5-HT, and/or dopamine located at the synapse in the depression model. Also the involvement of adult hippocampal neurogenesis will be studied.
References
- World Health Organization. Sixty-fifth world health assembly. 2012.
- Quanbin H, Jingzheng S, Chunfeng Q, Lina W, Hongxi XJ. Preparative isolation of hydrolysable tannins chebulagic acid and chebulinic acid from Terminalia chebula by high-speed counter-current chromatography, Chinese Medicine Laboratory, Hong Kong Jockey Club Institute of Chinese Medicine, Shatin NT. Hong Kong, China. 2006; 29: 1653-1657.
- Ta-Chen L, Feng-Lin H, Juel-Tang C. Antihypertensive activity of Corilagin and Chebulinic acid, tannins from Lumnitera recemosa. J Nat Prod. 1993; 56: 629 –632.
- Mishra V, Agrawal M, Onasanwo SA, Madhur G, Rastogi P, Pandey HP, et al. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 2013; 20: 506-511.
- Pfundstein B, Samy K Desouky E, Hull W, Haubner R, Gerhard E, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization quantitation and determination of antioxidant capacities. Phytochemistry. 2010; 71: 1132–1148.
- World Medical Association; American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002; 283: 281-283.
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977; 229: 327-336.
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985; 85: 367-370.
- Shimada T, Matsumoto K, Osanai M, Matsuda H, Terasawa K, Watanabe H. The modified light/dark transition test in mice: evaluation of classic and putative anxiolytic and anxiogenic drugs. Gen Pharmacol. 1995; 26: 205-210.
- Wei X, Yang J, Wu C. Anxiolytic effect of Baicalin in mice. Asian Journal of traditional medicines. 2006; 1: 3-4.
- Boissier J, Simon P. Dissociation de deux composantes dans le compartement d’investigation de la souris. Arch. Inter. Pharmacodyn. 1964; 147: 372-387.
- Taiwe G, Bum E, Dimo T, Talla E, Weiss E, Dawe A, et al. Antidepressant, Myorelaxant and Anti-Anxiety-Like Effects of Nauclea latifolia Smith (Rubiaceae) roots extract in Murine models. Int J Pharmacol. 2010; 6: 364-371.
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955; 48: 254-260.
- Kulkarni SK, Reddy DS. Animal behavioral models for testing antianxiety agents. Methods Find Exp Clin Pharmacol. 1996; 18: 219-230.
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005; 29: 571-625.
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl). 1987; 92: 180-185.
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007; 2: 322-328.
- EDDY NB. The relation of chemical structure to analgesic action. J Am Pharm Assoc Am Pharm Assoc. 1950; 39: 245-251.
- Pal D, Sannigrahi S, Mazumder UK. Analgesic and anticonvulsant effects of saponin isolated from the leaves of Clerodendrum infortunatum Linn. in mice. Indian J Exp Biol. 2009; 47: 743-747.