
Research Article
Ann Depress Anxiety.2015;2(1): 1042.
The Impact of Kolaviron (A Bioflavonoid of Garcinia Kola Seed) On Depression Status in Laboratory Rodents: Roles of Monoaminergic Systems
Onasanwo SA*, Ilenre KO and Faborode SO
Department of Physiology, University of Ibadan, Nigeria
*Corresponding author: Onasanwo SA, Neurosciences and Oral Physiology Unit, Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Nigeria
Received: January 27, 2015; Accepted: March 23, 2015 Published: March 31, 2015
Abstract
Garcinia kola seed is taken as a stimulant by the elderly people and traditionalists. Kolaviron is a biflavonoid from the seeds of Garcinia kola, and reports have shown that it may be the active principle for the analgesic, anti-inflammatory and psychoactive activities of Garcinia kola. Moreover, the aqueous extract of the seeds has shown an anxiolytic-like effect. Hence, this study was undertaken to investigate the antidepressant-like effect of kolaviron and probable mechanism(s) of action in laboratory rodents.
The antidepressant-like effect of kolaviron (50 – 200mg/kg, p.o.) was evaluated using the Forced Swimming Test (FST) and Tail Suspension Test (TST) in mice. Mice were pre-treated with prazosin (an a1-adrenoceptor antagonist), metergoline (5-HT2 receptor antagonist), and atropine (muscarinic cholinergic antagonist) before kolaviron (100 mg/kg p.o.) was administered, to evaluate the mechanisms involved in its antidepressant-like activity using the TST.
The results suggest that kolaviron possesses an antidepressant-like effect involving interactions with a1-adrenoceptor, 5-HT2 receptor and muscarinic acetylcholine receptors (mAChR).
Keywords: Antidepressant; Kolaviron; Garcinia kola; Forced swimming test; Tail Suspension Test; Monoamines
Introduction
Depression can become chronic or recurrent, and lead to substantial impairments in an individual’s ability to take care of his or her everyday responsibilities. On a yearly basis, about one million lives are lost due to suicide. This implies that about 3,000 suicidal deaths occur every day as a result of depression [1]. Depression has many symptoms including fatigue, impaired concentration, irritability, sleep disturbance, and somatization in addition to subjective experiences of nervousness, worry, and restlessness [2]. The discovery of antidepressant drugs in the 1950s led to the first biochemical hypothesis of depression, which suggested that impairment in the central Monoaminergic function was the major lesion underlying the disorder [3]. Basic research in all fields of neuroscience (including genetics) and the discovery of new antidepressant drugs have revolutionized our understanding of the mechanisms underlying depression and drug action. There is no doubt that the Monoaminergic system is one of the cornerstones of these mechanisms, but multiple interactions with other brain systems and the regulation of central nervous system function must also be taken into account. The cholinergic system is also implicated in depression. Evidence regarding altered cholinergic function in depression mainly comes from drug studies targeting nicotinic or muscarinic cholinergic receptors. These two types of cholinergic receptors are widely expressed in the brain and often co-expressed within neurons [4,5]. Their action is suggested to contribute to both mood and cognitive symptoms of depression. Similarly, there is little research providing direct evidence for a role of muscarinic receptors in the pathophysiology of major depressive disorder. Yet, pharmacological studies continue to recognize a potential for reducing depression associated symptoms via modulation of the muscarinic system [6]. For example, M1 metabotropic muscarinic receptors represent a viable target for amelioration of affective disorderassociated cognitive deficits, given their role in cognition [6]. M2 receptors were suggested to be involved in major depressive disorder by Single-Nucleotide Polymorphism (SNP) association studies [7,8]. This notion was further supported by a recent postmortem study, reporting decreased binding of M2and/or M4 muscarinic receptors in the dorsolateral prefrontal cortex of depressed subjects [9]. In spite of all the progress achieved so far, we must be aware that many open questions remain to be resolved in the future [3].
Plants extracts are some of the most attractive sources of new drugs, and have been shown to produce promising results for the treatment of depression [10]. Garcinia kola Heckel (Gultiferae) is a largely cultivated forest tree indigenous to sub-Saharan Africa [11]. Extracts of the plant have been traditionally used for ailments such as laryngitis, liver diseases and cough [12]. The seeds are used to prevent or relieve colic pains, cure head or chest colds and relieve cough [13]. The seed also has antiinflammatory, antimicrobial, antidiabetic and antiviral [14] as well as antiulcer properties [15]. Aqueous extract of Garcinia kola (Linn) has been shown to have an anxiolytic effect [16].
Kolaviron is a biflavonoid isolated from the seeds of Garcinia kola. It consists of Garcinia biflavonoid GB1, GB2 and Kola flavanone in ratio 2:2:1 [17]. Kolaviron has been extensively studied for its antihepatotoxic effects in various experimental models [18,19]. Kolaviron has been suggested to be the active principle for the analgesic and anti-inflammatory activities of Garcinia kola [20]. It may also inhibit acid secretion through proton pump inhibition and subsequently intensifying the defensive factors to a significant extent [21]. Despite the report of the anxiolytic potential of Garcinia kola, there are no reports about the use of kolaviron in the treatment of depression, neither are there reports about its mechanisms of action in the treatment of depression. Therefore, the objective of the present study was to investigate the antidepressant-like activity of kolaviron in different models in mice as well as to explore its possible mechanisms of action with focus on the monoaminergic system.
Materials and Methods
Plant materials
Fresh seeds of Garcinia kola were obtained locally in Ibadan, Oyo State, Nigeria. Peeled seeds (8.2 kg) were sliced, pulverized and airdried (25 – 28°C) for about 5 days.
Isolation of kolaviron
Extraction of kolaviron was achieved by the procedure previously described by Iwu [22] and as modified by Braide [23]. Blended Garcinia kola (8.2kg) was weighed and transferred into a glass container. Pure n-hexane (27L) were added, stirred at intervals of 2 hours, and allowed to stay for 72hours. The defatting process was repeated by adding another 5L of pure n-hexane. The n-hexane extracts collected after 72hours were added together filtered and concentrated using a rotary evaporator at the temperature of 400C and pressure of 600mm Hg.
The Garcinia kola marc was air-dried for 5hours and transferred back into a glass container. 27L of pure methanol was added to 1.5kg of the defatted Garcinia kola marc and allowed to stay for 72hours. The methanol extract was collected, filtered and concentrated using rotary evaporator at a temperature of 400C and a pressure of 600mm Hg. The concentrated methanol extract weighed 80g and was dissolved in 200ml of pure methanol before adding 200mL of distilled water, and then transferred into a separating funnel. Pure chloroform (400mL) was added before shaking and allowed to stay for 30 minutes for proper partitioning of the chloroform and methanol/water layers. The chloroform layer was collected and another 400mL of chloroform was added to the methanol/water extract. This process of fractionation was repeated 5 times and the chloroform fractions were pooled together and concentrated using a rotary evaporator at 40°C. The extract was further concentrated in a vacuum oven set at 40°C with a pressure of 600mm Hg.
Phytochemical screening
Kolaviron was screened for the presence or absence of various phytochemicals using standard procedures [24,25].
Drugs and treatment regimens
In the preliminary test, Graded doses of kolaviron (50 – 200mg/ kg, p.o.) and imipramine were administered to mice 1 hour prior to the Tail Suspension Test (TST). The most effective dose was used to evaluate the mechanisms of actions. For the mechanistic study, all drugs were freshly prepared before use after dissolving them in normal saline. The following drugs were used: metergoline (4mg/kg, i.p.) [26], prazosin (62.5 μg/kg, i.p.) [27], atropine (1 mg/ kg, i.p.) [28], imipramine hydrochloride (60mg/kg, p.o.) [29]. The drugs (antagonists) were administered to mice 15 minutes before administering kolaviron and 45mins later they were exposed to the test.
Experimental Animals
Female Swiss mice (22-25g) were used for this study. They were obtained from the certified dealers in Ibadan, Nigeria. Animals were housed in clean plastic cages under natural light and dark cycle. They were housed at room temperature with free access to water and mouse cubes (Ladokun Feeds Nig. Limited, Ibadan, Nigeria).
Depression models
Antidepressant activity of kolaviron was studied using two experimental procedures. These include the Forced Swimming Test and Tail Suspension Test. The preliminary test procedure was made up of 5 groups of 6 mice each. Group 1 was control and received vehicle (corn oil) only. Groups 2 - 4 were treated with kolaviron (50, 100 and 200mg/kg p.o., respectively) while Group 5 received imipramine (60mg/kg) as the reference drug. Test was carried out one hour after respective treatment.
Forced swimming test: This was performed as originally described by Porsolt and co-workers [30]. The apparatus consists of a clear plexiglass cylinder (20cm by 12cm) filled to a 15cm depth with water at 25±1°C. Experimental animals were pre-exposed to swimming environment for fifteen minutes each 24 hours prior to the test, and randomly assigned to five groups (n=6). One hour after treatment, animals were forced to swim for 6minutes and immobility time was recorded during the last five minutes using an automated stopwatch. The mice were assumed immobile when they made only movements necessary to keep their heads above water.
Tail suspension test: The Tail Suspension Test (TST) was performed as described by Steru and co-workers [31]. Mice were individually suspended 60cm above the surface of table on a metal rod with an adhesive tape placed 1cm away from the tip of the tail. Immobility duration was recorded in the last 5 minutes of a 6-minute test. Mice were considered immobile only when they hung passively and were completely motionless [32].
Elucidation of probable mechanism(s) of antidepressant activity of kolaviron in Tail Suspension Test: To assess the involvement of the serotonergic system in the antidepressant-like effect of kolaviron, mice were pre-treated with metergoline (4mg/ kg, i.p.), a non-selective 5-HT2 receptor antagonist, at a dose effective in blocking the in vivo effect induced by 5-HT2 receptor agonists in mice. To investigate the possible involvement of the noradrenergic system in the antidepressant-like effect of kolaviron, animals were pretreated with prazosin (62.5μg/kg, i.p.), an alpha-1-adrenoceptor antagonist. To investigate the involvement of cholinergic system in the antidepressant-like effect of kolaviron, animals were pre-treated with atropine (1mg/kg, i.p.). The test for elucidation of mechanism was made up of 5 groups of 6 mice each. Group 1 was control and received vehicle only. Group 2 was treated with kolaviron (effective dose, 100 mg/kg) while Groups 3 to 5 were treated with specified doses of some of the drugs stated above together with the effective dose of kolaviron.
Statistical analysis
Data were expressed as mean ±S.E.M. and analyzed by one-way Analysis Of Variance (ANOVA) followed by post hoc Newman- Keul’s test. The accepted level of significance was p<0.05. All statistical analyses were done by using Prism software, version 5 (GraphPad Soft Ware Inc., San Diego, CA, USA).
Results
Phytochemical screening
The results of the preliminary phytochemical screening revealed the presence of flavonoids, terpenoids, and alkaloids in kolaviron.
Antidepressant-like activity of kolaviron in the Forced Swimming Test
Oral administration of kolaviron (100mg/kg p.o.) produced significant increase in swimming activity (Figure 1b). Oral administration of Kolaviron (KV) had no significant effect in climbing activity (Figure 1c). Oral administration of kolaviron (50mg/kg p.o. at p<0.05; 100 and 200mg/kg p.o. at p<0.001) significantly decreased the duration of immobility in the FST (although not in a dose-related manner) when compared with the control. As expected, imipramine treatment (60mg/kg) produced significant (p<0.001) decrease in immobility time in comparison with kolaviron (50 and 200mg/kg p.o.) and control treatment except for 100mg/kg kolaviron. At 100 mg/kg, kolaviron produced significant (p<0.001) reduction in the duration of immobility with the least duration, which was almost similar to that produced following imipramine treatment (Figure 1a).
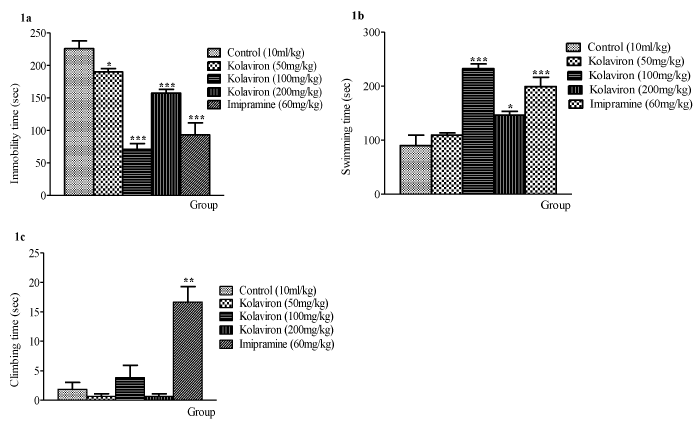
Figure 1: Effect of Kolaviron (KV) on (a) immobility (b) swimming, and (c) climbing time in Forced Swimming Test (FST). Values expressed as mean ±S.E.M. (n=6).
*p<0.05, **p<0.01, ***p<0.001 when compared with the control-treated group. Statistical level of significance analyzed by one-way ANOVA followed by Newman-
Keuls multiple comparison tests.
Antidepressant-like activity of kolaviron in the Tail Suspension Test
Oral administration of kolaviron (50 - 200mg/kg p.o.) decreased the duration of immobility in mice in tail suspension test. Reduced duration of immobility caused by kolaviron was significant at doses of 50mg/kg and 100mg/kg p.o. (p<0.05 and p<0.001, respectively) when compared with the control. Also, imipramine (60mg/kg) significantly (p<0.001) decreased immobility duration in mice (Figure 2).
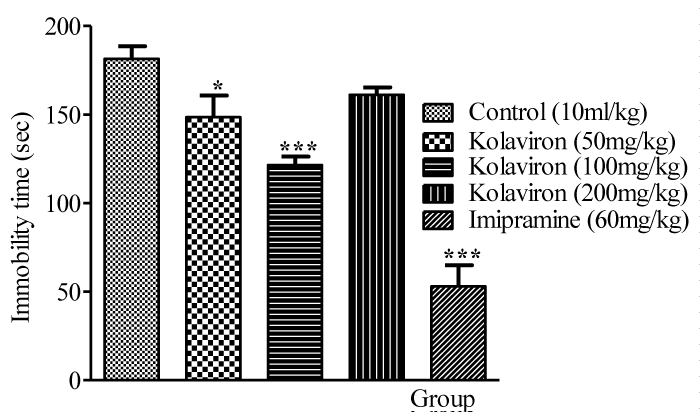
Figure 2: Effect of kolaviron on immobility in Tail Suspension Test (TST).
Values expressed as mean ±S.E.M, n=6. *p<0.05, ***p<0.001 versus control
group. Statistical level of significance analyzed by one-way ANOVA followed
by Newman-Keuls multiple comparison tests.
Elucidation of probable mechanism(s) of antidepressant activity of kolaviron in the Tail Suspension Test
The probable mechanism of action of kolaviron was investigated due to the antidepressant-like effect produced in the Tail Suspension Test. As shown in Figure 3, the pre-treatment of mice with metergoline (4mg/kg, i.p.) a 5-HT2 receptor antagonist, significantly increased the immobility time in comparison with kolaviron (100mg/ kg p.o.) only treated group. Pre-treatment with prazosin (62.5μg/ kg, i.p.), a a1-adrenoceptor antagonist, significantly prevented the decrease in the immobility time elicited by kolaviron (100mg/kg p.o.). In addition, a significant reduction in the immobility time elicited by kolaviron (100mg/kg) was also recorded. Pretreatment with atropine (1mg/kg i.p.), a muscarinic cholinergic antagonist, also reversed antiimmobility (p<0.05) effect of kolaviron.
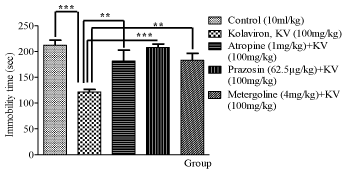
Figure 3: Affects of administration of atropine (1mg/kg i.p.), prazosin (62.5μg/
kg i.p.) and metergoline (4mg/kg i.p.) each 15 min before oral administration
of kolaviron (100mg/kg p.o.) on immobility in TST. Values expressed as mean
±S.E.M. (n=6). *p<0.05, **p<0.01, ***p<0.001 versus 100mg/kg treated.
Statistical level of significance analyzed by one-way ANOVA followed by
Newman-Keuls multiple comparison tests.
Discussion
Findings from this study showed that the kolaviron acts in a rather narrow range of doses following oral administration. In addition, this report suggests antidepressant-like effects of kolaviron in mice. The immobility displayed by mice when subjected to Forced Swimming Test (FST) is thought to reflect a state of despair or lowered mood, which is thought to reflect depressive disorders in humans [33]. In the regard of the use of female mice, reports have shown that variability in sex and the estrous cycle of female mice does not necessary appear to have a profound influence on their behavior in forced swimming test [34]. Kolaviron did not produce a dose-dependent reduction in immobility together with an increase in active swimming behavior with a significant change in climbing activity. The reduction of immobility after oral administration of kolaviron (100mg/kg p.o.) was almost similar to that observed after oral administration of imipramine (60mg/kg p.o.), the reference antidepressant drug. It is widely known that swimming activity is sensitive to serotoninergic compounds, such as the selective serotonin reuptake inhibitor (fluoxetine), whereas the climbing behavior is sensitive to tricyclic antidepressants like imipramine [35]. Taking this into consideration, the results obtained in this study suggested the implication of the serotoninergic pathway in the antidepressant effect of kolaviron.
The Monoaminergic hypothesis of depression postulates that the major neurochemical process in depression is the impairment of Monoaminergic neurotransmission and the concomitant decrease of extracellular concentration of nor adrenaline and/or serotonin [36]. In addition, these neurotransmitter systems are also involved in the expression of an antidepressant-like effect in behavioral despair models of depression [37]. To determine the possible mechanism of antidepressant-like activity of kolaviron, some experimental assays were conducted to explore the involvement of 5-HT2, a1 adrenergic receptors and cholinergic receptors. Depression is associated with a hypo function of the noradrenergic system, and some antidepressants such as nor adrenaline reuptake inhibitors or monoamine oxidase inhibitors act by increasing the synaptic availability of noradrenaline [38]. There is compelling evidence for the role of a1-adrenoceptors in the mechanism of action of antidepressant agents [39]. It was shown that the antidepressant-like action of desipramine was blocked by the pre-treatment of mice with the a1-adrenoceptor antagonist, prazosin [39].
In this study, pre-treatment with metergoline (a non-selective 5-HT2 receptor antagonist) was able to significantly reverse the decrease in immobility time induced by kolaviron. This finding suggests that serotonergic system plays a role in the antidepressantlike effect of kolaviron. Hypo function of the noradrenergic system was linked with depression [38]. Hence, adrenergic antagonist was used in our study to explore the role or involvement of noradrenergic system in the antidepressant-like effect of kolaviron. The results showed that the pretreatment with prazosin (a1-adrenergic antagonist) reversed the antidepressant-like effect of kolaviron, indicating that kolaviron may exert its effect in the tail suspension test by interacting with a1- adrenoceptors. Reversal of the antidepressant-like effect of kolaviron after pretreatment with atropine (muscarinic cholinergic antagonist) also suggests the involvement of the cholinergic system.
Findings from this study showed that kolaviron (a biflavonoid of Garcinia kola) possesses antidepressant-like properties which may be mediated via interactions with serotonin (5-HT2) receptor, a1- adrenoceptor and muscarinic acetylcholine receptor.
In conclusion, this study has been able to show a possible involvement of kolaviron in mental disorder in animal models of depression. Specifically, this study has provided information about the antidepressant properties of kolaviron, which is specific compound from Garcinia kola plant. It has also been able to show probable mechanisms via which it potentiates its antidepressant properties. This may help in the development of a better drug that may be useful in the treatment of depressive illness.
Further neurochemical studies are necessary to elucidate the influence of kolaviron on other monoamine systems (e.g., dopaminergic system and other sub-types of serotonin), which are involved in the pathophysiology of depression. Although, it has been reported that aqueous extract of Garcinia kola (linn) shows an anxiolytic effect with the use of malnourished mice [16], it would also be necessary to investigate to see whether or not kolaviron possesses an anxiolytic effect.
Acknowledgment
The authors are grateful to Mr. Tosin Ale, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Ibadan, Ibadan for his technical assistance during the period of this study.
References
- World Health Organization. World Suicide Prevention Day. 2012.
- Ninan PT. The functional anatomy, neurochemistry, and pharmacology of anxiety. J Clin Psychiatry. 1999; 22: 12-17.
- Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002; 4: 7-20.
- Schroder H, Giacobini E, Struble RG, Luiten PG, van der Zee EA, Zilles K, et al. Muscarinic cholinoceptive neurons in the frontal cortex in Alzheimer's disease. Brain Res Bull. 1991; 27: 631-636.
- van der Zee EA, Streefland C, Strosberg AD, Schroder H, Luiten PG. Colocalization of muscarinic and nicotinic receptors in cholinoceptive neurons of the suprachiasmatic region in young and aged rats. Brain Res. 1991; 542: 348-352.
- Scarr E. Muscarinic receptors in psychiatric disorders - can we mimic 'health'? Neurosignals. 2009; 17: 298-310.
- Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002; 114: 527-529.
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004; 13: 1903-1911.
- Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. Decreased muscarinic receptor binding in the prefrontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord. 2009; 116: 184-191. Akhondzadeh S, Kashani L, Fotouhi A, Jarvandi S, Mobaseri M, Moin M, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27: 123-127.
- Hutchinson J, Dalziel JM. Flora of West Tropical Africa. 2nd edn. HMSO; London. 1956; 11: 295.
- Ayensu ES. Medical Plants of West Africa. Reference Publication, Inc. Michigan, USA. 1978; 163.
- Iwu MM. Handbook of African medical plants. Bola Raton: CRC ress Inc. 1993; 223-224.
- Iwu MM. In Plant flavonoids in Biology and Medicine. Cody V, Middleton E, Harbone JB, editors. Ala R. Liss. New York. 1986; 485.
- Ibironke GF, Olaleye SB, Balogun O, Aremu DA. Effect of diets containing seeds of Garcinia kola (Heckel) on gastric acidity and experimental ulceration in rats. Phytother Res. 1997; 11: 312-313.
- Ajayi SA, Nwoha PU. The use of elevated plus maze to study the effects of aqueous extract of Garcinia kola (linn) on the anxiety status of malnourished mice. Rev. Electronic Journal of Biomedicine. 2011; 2: 63-67.
- Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol. 1990; 42: 290-292.
- Akintonwa A, Essien AR. Protective effects of Garcinia kola seed extract against paracetamol-induced hepatotoxicity in rats. J Ethnopharmacol. 1990; 29: 207-211.
- Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron--a Garcinia kola seed extract. Food Chem Toxicol. 2000; 38: 535-541.
- Olaleye SB, Farombi EO, Adewoye EO, Onasanwo SA, Owoyele BV, Elegbe RA. Analgesic and anti-inflammatory effects of kolaviron (a Garcinia kola seed extract). Afr J Biomed Res. 2000; 3: 163.
- Onasanwo SA, Singh N, Olaleye SB, Palit G. Anti-ulcerogenic and proton pump (H+, K+ ATPase) inhibitory activity of Kolaviron from Garcinia kola Heckel in rodents. Indian J Exp Biol. 2011; 49: 461-468.
- Iwu MM. Antihepatoxic constituents of Garcinia kola seeds. Experientia. 1985; 41: 699-700.
- Braide VP. Pharmacological effect of chronic ingestion of Garcinia kola seeds in rats. Phytotherapy Res. 1990; 4: 39-41.
- Trease GE, Evans WC. Pharmacognosy. 13th edn. Bailliere Tindall, London. 1989; 176-180.
- Sofowora A. Medicinal plants and Traditional Medicine in Africa. Spectrum Books, Ibadan. 1993; 150.
- Gigliucci V, Buckley KN, Nunan J, O'Shea K, Harkin A. A role for serotonin in the antidepressant activity of NG-Nitro-L-arginine, in the rat forced swimming test. Pharmacol Biochem Behav. 2010; 94: 524-533.
- Gu L, Liu YJ, Wang YB, Yi LT. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J Ethnopharmacol. 2012; 139: 780-787.
- Liebenberg N, Wegener G, Harvey BH, Brink CB. Investigating the role of protein kinase-G in the antidepressant-like response of sildenafil in combination with muscarinic acetylcholine receptor antagonism. Behav Brain Res. 2010; 209: 137-141.
- Chatterjee M, Verma P, Maurya R, Palit G. Evaluation of ethanol leaf extract of Ocimum sanctum in experimental models of anxiety and depression. Pharm Biol. 2011; 49: 477-483.
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978; 47: 379-391.
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985; 85: 367-370.
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005; 29: 571-625.
- Detke MJ, Rickels M, Lucki I. Active behaviours in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995; 121: 66-72.
- Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav. 1991; 49: 69-72.
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965; 122: 509-522.
- Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28: 435-451.
- Millan MJ1. The role of monoamines in the actions of established and "novel" antidepressant agents: a critical review. Eur J Pharmacol. 2004; 500: 371-384.
- Montgomery SA. Predicting response: noradrenaline reuptake inhibition. Int Clin Psychopharmacol. 1999; 14 Suppl 1: S21-26.
- Danysz W, Kostowski W, Kozak W, Hauptmann M. On the role of noradrenergic neurotransmission in the action of desipramine and amitriptyline in animal models of depression. Pol J Pharmacol Pharm. 1986; 38: 285-298.