
Research Article
Ann Depress Anxiety. 2016; 3(2): 1080.
Combined Caffeine and Lecithin Induces Anxiogenic-Like Behaviours via Oxidative Stress
Owoyele BV¹, Toluhi AM¹, Oyewole AL¹*, Imam- Fulani AO¹ and Yakubu MT²
¹Departments of Physiology, University of Ilorin, Nigeria
²Department of Biochemistry, University of Ilorin, Nigeria
Corresponding author: Oyewole AL, Departments of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, PMB 1515, Ilorin, Nigeria
Received: July 19, 2016; Accepted: September 05, 2016; Published: September 13, 2016
Abstract
Caffeine and lecithin were documented with many different contradictory effects in the body. In these studies, we set to investigate possible ameliorating effect of lecithin on anxiogenic effect of caffeine, possible mechanism of combined caffeine and lecithin on anxiety-like behaviours as well as potential anxiety biomarker. Thirty-six adult male Wistar rats of an average weight of 140- 145g was divided into six groups (1 - 6) of six rats each and were used for these studies. Groups 1, 2 and 3 were given normal saline (10ml/kg), lecithin (20mg/kg) and caffeine (10mg/kg) respectively, while Groups 4, 5 and 6 were treated with combined caffeine and lecithin (10mg/kg + 20mg/kg), (5mg/kg + 30mg/kg) and (15mg/kg + 10mg/kg) respectively. All treatments were done orally via oral cannula, once daily and repeated for 14 days. Two hours after the last treatment, anxiety-like behaviours were evaluated on open field maze. Animals were finally sacrificed under ketamine (50mg/kg); blood and brain were harvested to quantify lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G6PD). Histological study on the amygdala was done using H&E. Combined caffeine and lecithin at the doses used significantly (p<0.05) decreased locomotor and exploratory behavioural parameters (anxiogenic effects), caused both significant increase and decrease of blood and brain LDH and significantly (p<0.05) decreased in the blood and the brain G6PD compared to the control group. Histological studies demonstrated normal cell morphology and distribution in all groups. In conclusion, combined caffeine and lecithin decreases G6PD leading to decrease antioxidants and increase oxidative stress which in turn stimulate anxiogenic-like behaviours. With the decreasing pattern of G6PD recorded in this study, G6PD might be a potential biomarker for generalized anxiety disorders.
Keywords: Anxiety; Behaviour; Biomarker; Caffeine; Lecithin; Rat
Introduction
Anxiety disorder is a rapidly emerging global health challenge among various psychiatric illnesses [1]. The disorders may present as post-traumatic stress disorders, obsessive compulsive disorders, panic disorders and phobias [2]. Association between suicidal attempts and anxiety disorders has been well proven by increasing clinical evidence [3]. In rodents, anxiety can be evaluated from anxiety-like behaviour display on mazes such as open field test, elevated plus maze test [4] and hole-board test [5]. Despite steady increase in the numbers of anxiolytic drugs use for the treatment of anxiety disorders, the prevalence rate of these disorders persistently remain high [1], this might be because of inconsistent efficacy of current pharmacotherapy [6]. Ethno-pharmacology study targeting for treatment of anxiety disorder is thus always welcome. In fact, this study aimed to search for beneficial or harmful effects of combined caffeine and lecithin on anxiety-like behaviour.
Caffeine is a naturally occurring central stimulant and a secondary metabolite from plants such as Coffea arabica and C. canephora [7,8]. Apart from the ones consumed from sweet, energy drinks, food and drugs [9], approximately 2.5 billion cups (30 mL/ cup) of coffee is consumed per day [10]. In the body, caffeine acts as a nonselective antagonist of macromolecular structures, known as adenosine receptor [11]. Through its receptor and possibly many others, caffeine was documented to interfere with testicular growth and steroidogenesis during puberty [12], regulate oxidative balance in the body [8], disturb the renin-angiotensin system in chronic prenatal exposure [13], and decrease locomotor activity and memory in chronic exposure in pregnant rats [14]. Clinically, caffeine intoxication and overdose present with agitation, anxiety, headache, fever, dizziness, tinnitus, seizures, arrhythmia, hypertension and sometime hypotension [15,16]. Depending on the doses administered in rodents, caffeine has both anxiolytic and anxiogenic effects [17,18]. Information on the effects of combined caffeine and lecithin on anxiety disorder or anxietic-like behaviour remain elusive in the literature. Thus, we intend to provide this information in these studies.
Lecithin is a mixture of lipids, (glycolipids, phospholipids and triglycerides), that can be obtained from natural sources such as egg yolk and soy [19]. In fact, it was first isolated from egg yolk in 1846 by Theodore Gobley [20]. Depending on the origin, lecithin differs in composition and polar head groups thus making its physico-chemical properties vary [21]. In purity form, it is sold as food supplement for medical purposes [22]. As emulsifier and lubricant, lecithin is used in food or pharmaceutical industry [19]. Also, lecithin consumption is documented as safe for humans and it is also well-tolerated in different organisms because it occurs naturally as a component of cell membrane [19,23]. Studies showed that lecithin has positive effects on brain functions, memory improvement, cholesterol metabolism and physical activity [24,25]. Increased physical activity such as grooming, rearing and locomotor activity on open field maze is ascribed as anxiolytic related behaviour [26].
Lactate Dehydrogenase (LDH) is a cytoplasmic cellular enzyme present essentially in all major organ systems. Its serum level is abnormally high in a number of disorders thus it is used in monitoring activity of pathological conditions in damaged or inflammated cells [27]. Because of its wide presence in the body, total serum LDH test is highly sensitive but not specific. It is documented to be released into the blood after cell death induced by certain drugs, ischaemia, dehydration, chemical poisonings and exposure to bacterial toxins [28,29]. In a cardiomyopathy-induced anxiety in mice, LDH increased while the anxiety-like effects induced by doxorubicin decreased follow escitalopram treatment [30].
Glucose-6-Phosphate Dehydrogenase (G6PD) is an X-linked cytoplasmic enzyme that catalyses first oxidative phase of the hexose monophosphate pathway [31]. This rate-limiting enzyme is commonly called housekeeping enzyme due to its Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-generating activity [32] during the conversion of glucose-6-phosphate to 6-phosphogluconolactone. NADPH is essential for many reductive biosynthetic pathways and redox regulation [33]. Deficiency of G6PD is not uncommon in human enzymopathy. In fact, more than 400 million people across the globe have G6PD deficiency resulting from over 186 mutation in some of over 400 variant G6PD alleles reported in the human population [33,34]. Clinically, G6PD deficiency presents with hemolytic syndromes [35], neonatal jaundice [36] and oxidative stress [31].
Materials and Methods
Animal’s preparation
Thirty-six male albino Wistar rats weighing 140–145g were housed under conducive environmental conditions in the Animal Facility of College of Health Sciences, University of Ilorin. The rats were obtained from Department of Biochemistry, University of Ilorin, Nigeria. Animals were offered food and water ad libitum and were subsequently randomly distributed into six group (n=6) of six rats with the following label: Group 1 (Normal saline 0.9%,10ml kg- 1), Group 2 (20mg kg-1 of Lecithin), Group 3 (10mg kg-1 of Caffeine), Group 4 (10mg kg-1 of Caffeine + 20mg kg-1 of Lecithin), Group 5 (5mg kg-1 of Caffeine + 30mg kg-1 of Lecithin), Group 6 (15mg kg-1 of Caffeine + 10mg kg-1 of Lecithin). All studies were performed in accordance with the University of Ilorin ethical guidelines for animal studies.
Drug preparation
Crystalline powder of Caffeine Anhydrous (produced by CSPC Innovation Pharma, China) and Lecithin (produced by Tianjin Tianshi Biological Development Co. Ltd., China) were used for this study. Stock solutions of both drugs were prepared separately by dissolving 1g of each drug in 10ml of normal saline (produced by DANA Plc.). All treatments were done orally, once daily and were repeated for two weeks (14 days). Two hours after the final treatment on the fourteenth day, five animals from each group were separately evaluated for anxiety-like behaviour on open field maze. The animals were finally sacrificed under ketamine (produced by Rotexmedica, Trittau, Germany), brain and blood (via cardiac puncture) were collected to quantify the level of lactate dehydrogenase and glucose-6- phosphate dehydrogenase. The sixth animal from each group was also sacrificed and amygdala was harvested from the brain for histological study.
The histological study was carried out using heamatoxylin and eosin stain. This was done to investigate possible changes in the structure and cells distribution of the amygdala in the treated animals compared with the control. This is because over a period of time, drugs-induced physiological changes are sometime accompanied with structural changes in the effectors or control centers.
Behavioural test
Open field maze: The open field maze is more advantageous in the evaluation of anxiety-like behaviour because it allows simultaneous measurement of different events. Test was carried out as described previously [26] where behavioural parameters such as rearing, grooming and ambulation were used as anxiety index. Rearing is a behaviour in which rodents stand on their hind limbs with their fore limbs raised into the air and/or placed on the wall of their cages or mazes. Grooming is however a caressing attitude of rodents on their body. This behavior is characterized by the animal cleaning its paws, tail and body with its mouth as well as using its fore paws to wipe its face and head. Ambulation, otherwise known as line crossing, is the movement of the rodent from one cell of the maze to another. The total of each event was measured by visualizing 5 minutes recorded video of the animals’ activities.
Biochemical assays of serum and brain LDH and G6PD
Blood was collected via cardiac puncture in a plain bottle for the determination of serum lactate dehydrogenase and glucose-6- phosphate dehydrogenase level. Briefly, this was done by centrifuging the blood at 3000 × g for 15 min and the serum was extracted using 200μL micropipette. Lactate dehydrogenase and glucose-6-phosphate dehydrogenase level were measured by standard enzymaticcolorimetric method using enzymatic kits produced by Randox Laboratories Limited, UK. The minimum detectable concentration for G6PD is 156 UI-1 and 55.1 UI-1 for LDH.
Statistical analysis
All statistical analyses were done using SPSS 16 (SPSS Inc., Chicago, IL, USA). Results were presented as mean ± Standard Error of Mean (S.E.M.) for five (5) rats per group. Statistical difference between the control and the treated groups was by one way ANOVA and post hoc multiple comparisons were done using Duncan option. Statistically significant differences were accepted at p < 0.05.
Results
The results of combined caffeine and lecithin for all responses recorded on open field maze are presented in (Table 1). Also, serum and brain lactate dehydrogenase with glucose-6-phosphate dehydrogenase were determined, analyzed and presented as shown in (Tables 2 & 3) respectively. Group 1 (Normal saline), Group 2 (Lecithin only) and Group 3 (Caffeine only) were compared separately with combined caffeine and lecithin Groups 4, 5 and 6. While ap<0.05 was ascribed to group with significant difference compared to Group 1, bp<0.05 and cp<0.05 were ascribed to group with significant difference compared to Groups 2 and 3 respectively.
Effects of combined lecithin and caffeine on anxiety indices on the open field maze
The time spent on grooming and ambulatory activity on the open field maze was significantly (ap<0.05) decreased in all the treated groups compared to the control (Group 1). While rearing activity decreased significantly (ap<0.05) in Groups 2 and 3 compared to Group 1, combined caffeine and lecithin Groups 4, 5 and 6 were not significantly difference (Table 1).
In the multiple comparison for these activities, there was no significant (b,cp<0.05) difference in grooming and ambulatory activity when Groups 2 and 3 were compared with combined caffeine and lecithin Groups 4, 5 and 6. Post Hoc analysis however showed that there was significant (b,cp<0.05) decrease in rearing activity (Table 1) in Groups 5 and 6 compared to Groups 2 and 3.
Effects of combined lecithin and caffeine on lactate dehydrogenase level
Serum lactate dehydrogenase decrease significantly (ap<0.05) in Groups 2, 5, and 6 compared with Group 1 but it was significantly (ap<0.05) increased in Groups 3 and 4. In the multiple comparison, while serum lactate dehydrogenase was significantly (bp<0.05) increased in all the treated groups compared with Group 2, the enzyme was significantly (cp<0.05) decreased in all the treated groups compared with Group 3 (Table 2).
Brain lactate dehydrogenase also decrease significantly (ap<0.05) in all the treated groups compared with Group 1 except in the Group 6 where a significant (ap<0.05) increase was recorded. Compared with Group 2, there was a significant (bp<0.05) increase in brain lactate dehydrogenase in the Groups 3 and 6 but there was a significant (bp<0.05) decrease in brain lactate dehydrogenase in Group 4. Compared with Group 3, caffeine only, brain lactate dehydrogenase significantly (cp<0.05) decrease in Groups 2, 4 and 5 but significantly (cp<0.05) increase in Group 6 (Table 2).
Effects of combined lecithin and caffeine on Glucose-6- Phosphate dehydrogenase level
With the exception of Group 5, quantified serum glucose-6- phosphate dehydrogenase showed a significant (ap<0.05) decrease in all the treated groups compared with Group 1. Compared with Group 2 however, Group 3 and 6 were significantly (bp<0.05) decreased while Group 5 was significant (p<0.05) increased. Also, serum glucose-6-phosphate dehydrogenase of all the treated group was significantly (cp<0.05) increased when compared with Group 3, the caffeine treated group (Table 3).
On the other hand, brain glucose-6-phosphate dehydrogenase was significantly (ap<0.05) decreased in all the treated groups compared with Group 1 except in Group 4 where there was no significant (ap<0.05) difference. When compared with Group 2 however, there was a significant (bp<0.05) decrease in brain glucose-6-phosphate dehydrogenase in Group 3 and 6 but it was significantly (bp<0.05) increased in group 4. Brain glucose-6-phosphate dehydrogenase was significantly (cp<0.05) increased in all groups compared with Group 3 (Table 3).
Effects of combined lecithin and caffeine on neural cells in the amygdala
Neural cells in the amygdala of the control stained normally, cell morphology and distribution appearred to be normal (Figure 1). In the Group 2 treated with lecithin only, neural cells staining and distribution is smilar to the control but the neural cells appeared to be enlarged signifying possible increased neural activities. In the caffiene (Group 3) only treated group, neural cells appeared to increase in number compared to Group 1. Staining of this cells was normochromic but very few of the neural cells were hypertrophied. Groups 4 and 5 showed hypochromic to the stain. Neural cells appearred to be more numerous than in group 1 but many showed faintly with more perinuclear spaces. Group 6 stained hyperchromic with obvious increase in the number of neural cells compared to Group 1. Cells morphology was normal and neural cells distribution was more thorough compared to Group 1 (Figure 1).
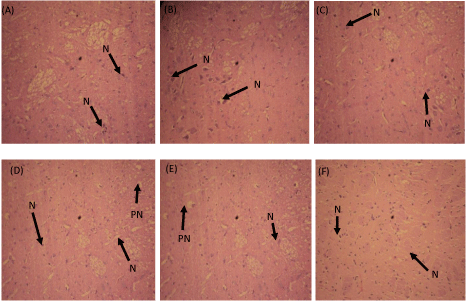
Figure 1: Effects of combined lecithin and caffeine on neural cells in the
amygdala (X 160; H & E stain).
N: Nucleus; PN: Perinuclear space; (A) - Group 1 (Normal saline, 10ml/kg);
(B) - Group 2 (Lecithin, 20mg/kg); (C) - Group 3 (Caffeine, 10mg/kg); (D) -
Group 4 (Caffeine and Lecithin, 10mg/kg & 20mg/kg); (E) - Group 5 (Caffeine
and Lecithin; 5mg/kg & 30mg/kg); Group 6 (Caffeine and lecithin, 15mg/kg
& 10mg/kg).
Discussion
Anxiety disorder is a group of mental disorders usually presenting with phobias of future events. The most common is the subthreshold generalized anxiety, a recurrent and impairing disease with high comorbidity rates of other mood disorder that presently claims significant healthcare resource [37] worldwide. With this global economy burden of subthreshold generalized anxiety, new prophylactic and anaphylactic measures and studies are always welcomed.
When used alone, lecithin caused significant decrease in LDH (Table 2) and G6PD (Table 3) as well as inducing anxiogenic related behaviour (Table 1). These stated effects of lecithin seem missing in the literature. Role of lecithin in cells viability [38] was emphasized in our histological study of amygdala (Figure 1(B)) where the neural cells were well hypertrophied. Caffeine alone treatment (Group 3) also induced anxiogenic-related behaviour (Table 1), decreased G6PD (Table 3), decreased brain LDH but increased serum LDH (Table 2). Anxiogenic effects of caffeine had been reported [39], but its effects on LDH and G6PD as stated in this study remain elusive in the literature. It is interesting to know that despite lecithin and caffeine have similar effects on anxiogenic related behaviour, G6PD and LDH when used separately as stated herein, facts in the literature showed that both substances have contradictory effects. For instance, while lecithin mitigate oxidative stress [40], caffeine induces oxidative stress [41,42]. Also, lecithin decreases serotonin binding and availability while caffeine increases serotonin availability [43].
Group
Ambulatory
Latency (s)
Rearing
Latency (s)
Grooming
Latency (s)
Group 1
13.40±2.88
12.40±1.34
21.00±5.79
Group 2
5.40±1.52a
16.80±3.70a
12.60±1.34a
Group 3
6.00±4.18a
18.80±3.70a
10.40±5.79a
Group 4
6.80±3.19a
15.40±2.88
10.60±2.97a
Group 5
7.40±2.19a
12.40±1.14bc
12.20±2.68a
Group 6
7.20±1.92a
12.80±1.92bc
10.00±0.71a
Each value is the mean ± S.E.M. of 5 Wistar rats; ap < 0.05 compared with Group 1; bp < 0.05 compared with Group 2 and cp < 0.05 compared with Group 3; ANOVA. Group 1 (Normal saline, 10ml/kg); Group 2 (Lecithin, 20mg/kg); Group 3 (Caffeine, 10mg/kg); Group 4 (Caffeine and Lecithin, 10mg/kg & 20mg/kg); Group 5 (Caffeine and Lecithin, 5mg/kg & 30mg/kg); Group 6 (Caffeine and lecithin, 15mg/kg & 10mg/kg).
Table 1: Effects of combined lecithin and caffeine on anxiety indices on the open field maze.
Group
Serum (UI-1)
Brain (UI-1)
Group 1
1123.40±67.90
924.40±46.27
Group 2
459.80±91.18ac
495.60±47.15ac
Group 3
1306.20±93.77ab
670.00±186.57ab
Group 4
1296.80±19.49ab
241.40±61.43abc
Group 5
544.20±34.65ac
526.20±27.09ac
Group 6
995.80±6.87abc
1003.40±4.82bc
Each value is the mean ± S.E.M. of 5 Wistar rats; ap < 0.05 compared with Group 1; bp < 0.05 compared with Group 2 and cp < 0.05 compared with Group 3; ANOVA. Group 1 (Normal saline, 10ml/kg); Group 2 (Lecithin, 20mg/kg); Group 3 (Caffeine, 10mg/kg); Group 4 (Caffeine and Lecithin, 10mg/kg & 20mg/kg); Group 5 (Caffeine and Lecithin, 5mg/kg & 30mg/kg); Group 6 (Caffeine and lecithin, 15mg/kg & 10mg/kg).
Table 2: Effects of combined lecithin and caffeine on lactate dehydrogenase (LDH) level.
Group
Serum G6P (UI-1)
Brain G6P (UI-1)
Group 1
4502.40±170.03
4637.00±160.84
Group 2
4051.40±63.49ac
4166.20±26.89ac
Group 3
505.40±81.22ab
471.20±260.14ab
Group 4
4091.80±212.43ac
4617.00±128.00bc
Group 5
4246.80±130.99bc
4159.40±22.76ac
Group 6
3378.40±13.40abc
3331.20±15.09abc
Each value is the mean ± S.E.M. of 5 Wistar rats; ap < 0.05 compared with Group 1; bp < 0.05 compared with Group 2 and cp < 0.05 compared with Group 3; ANOVA. Group 1 (Normal saline, 10ml/kg); Group 2 (Lecithin, 20mg/kg); Group 3 (Caffeine, 10mg/kg); Group 4 (Caffeine and Lecithin, 10mg/kg & 20mg/kg); Group 5 (Caffeine and Lecithin, 5mg/kg & 30mg/kg); Group 6 (Caffeine and lecithin, 15mg/kg & 10mg/kg).
Table 3: Effects of combined lecithin and caffeine on glucose-6-phosphate dehydrogenase level.
At the doses used in this study, combined caffeine and lecithin significantly decrease locomotor and exploratory activities on open field maze except the time spent on rearing activities which recorded no significant difference (Table 1). The results as presented in this study showed that combined caffeine and lecithin has anxiogenic effects thus demonstrating the possibility of inducing anxiety disorder or decreasing its threshold in a normal people.
To substantiate if the observed effects of combined lecithin and caffeine was initiated by apoptosis of the amygdala neural cells or that of the other part of the body, LDH, a nonspecific marker for apoptosis was quantified in the serum and brain. Except in Group 4 where there was significant increase in serum LDH and Group 6 where significant increase was recorded in the brain LDH level, serum and brain LDH levels decreased significantly in all the group treated with varying doses of combined lecithin and caffeine compared to the control. Corroborating this with histological study of the amygdala using H&E stain, photomicrograph revealed no sign of cell death but increased neural cells count thus increased cellular activity in Group 6 (Figure 1(F)) compared to the control (Figure 1(A)). Increased LDH measured in this group was thus not from apoptosis of amygdala cells but probably from other area of the brain which was not part the present studies. We could not find any correlation between the anxiogenic-like behaviour and LDH measured thus contradicting a previous claim that LDH increased as anxiety-like effects induced by doxorubicin decreased following escitalopram treatment [30].
Serum and brain G6PD level decreased significantly in all the treated groups except in Group 4 where brain G6PD was not significantly different from normal control, Group 1. This decrease is more obvious in the group treated with only caffeine compared to other groups. It was observed that decrease G6PD followed decrease in exploratory behaviour in groups treated with combined lecithin and caffeine. We thus deduce that with further studies, brain G6PD level might serve as a useful biomarker for the diagnosis or monitoring of treatment in generalized anxiety disorder.
Combined lecithin and caffeine induced anxiogenic related behaviour via the serotonergic and oxidative pathways (Figure 2). In the oxidative pathway, combined lecithin and caffeine decreased G6PD which led to decrease antioxidants [33,44] within the CNS or outside the CNS. Decrease antioxidants leads to increase oxidative radicals thus oxidative stress which in turn leads to anxiogenic related behaviour [45]. In the serotonergic pathway, both lecithin and caffeine are known to interact with serotonin and its receptors [43,46,47]. Serotonergic system originates from raphe nuclei and it projects to corticolimbic structures such as amygdala, frontal cortex and hippocampus where it plays a major role in the control of mood and emotional behaviour [48]. Excessive 5HT stimulation of this system may cause anxiety [1]. While we suspected that lecithin blocks 5-HT1B/D receptor centrally to induce anxiogenic-like behaviour [49], caffeine is known to activate 5-HT2A receptor that initiate oxidative stress via G-protein, phospholipase C, classical PKC, NADPH oxidase and eventually induce anxiogenic-like behaviour [50]. Also, caffeine has the ability to increase central serotonin level [43] which can lead to cellular oxidative stress [51] and finally induce anxiogenic related behaviour [45].
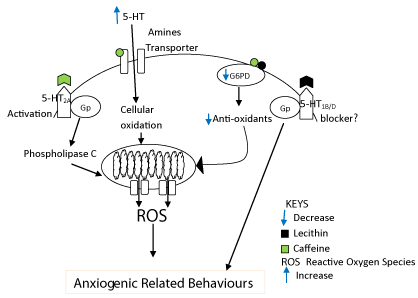
Figure 2: Illustration on cellular mechanism of combined lecithin and caffeine
treatment.
Conclusion
This study was able to establish that the use of caffeine and lecithin separately or combined induces anxiogenic-like behaviour in rats possibly through oxidative stress.
References
- Gupta D, Radhakrishnan M, Kurhe Y. Anxiolytic-like effects of alverine citrate in experimental mouse models of anxiety. European Journal of Pharmacology. 2014; 742: 94-101.
- Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model: advances and strategies in animal modeling of anxiety and depression? Behav Brain Res. 2007; 179: 1-18.
- Capron DW, Fitch K, Medley A, Blagg C, Mallott M, Joiner T. Role of anxiety sensitivity sub factors in suicidal ideation and suicide attempt history. Depress Anxiety. 2012; 29: 195–201.
- Estrela Dda C, da Silva WAM, Guimaraes ATB, Mendes BdeO, Castroe AL daS, Torres IL da S, et al. Predictive behaviors for anxiety and depression in female Wistar rats subjected to cafeteria diet and stress. Physiology & Behavior. 2015; 151: 252–263.
- Armario A, Hernandez J, Bluethmann H, Hidalgo J. IL-6 deficiency leads to increased emotionality inmice: evidence in transgenic mice carrying a nullmutation for IL-6. J Neuroimmunol. 1998; 92: 160–169.
- Jindal A, Mahesh R, Kumar B. Anxiolytic-like effect of linezolid in experimental mouse models of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 40: 47–53.
- Yamamoto T, Yoshizawa K, Kubo SI, Emoto Y, Hara K, Waters B, et al. Autopsy report for a caffeine intoxication case and review of the current literature. J Toxicol Pathol. 2015; 28: 33–36.
- Pohanka M. Caffeine alters oxidative homeostasis in the body of BALB/c mice. Bratisl Lek Listy. 2014; 115: 699-703.
- Banerjee P, Ali Z, Levine B, Fowler DR. Fatal caffeine intoxication: a series of eight cases from 1999 to 2009. J Forensic Sci. 2014; 59: 865–868.
- Heckman MA, Weil J, Gonzalez de ME. Caffeine (1, 3, 7-trimethylxanthine) in foods:a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010; 75: 77–87.
- Potenza RL, Armida M, Rerrante A, Pezzola A, Matteucci A, Puopolo M, et al. Effects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosis. J Neurosci Res. 2013; 91: 585–592.
- Park M, Choi Y, Choi H, Yim JY, Roh J. High Doses of Caffeine during the Peripubertal Period in the Rat Impair the Growth and Function of the Testis. International Journal of Endocrinology. Article ID. 2015; 368475: 9.
- Serapia o-Moraes DF, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA, Faria TS. Maternal caffeine administration leads to adverse effects on adult mice offspring. Eur J Nutr. 2013; 52: 1891–1900.
- Soellner DE, Grandys T, Nun ez JL. Chronic prenatal caffeine exposure impairs novel object recognition and radial arm maze behaviors in adult rats. Behav Brain Res. 2009; 205: 191–199.
- Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005; 153: 67–69.
- Rudolph T, Knudsen K. A case of fatal caffeine poisoning. Acta Anaesthesiol Scand. 2010; 54: 521–523.
- Ardais AP, Borges MF, Rocha AS, Sallaberry C, Cunha RA, Porciuncula LO. Caffeine triggers behavioral and neurochemical alterations in adolescent rats. Neuroscience. 2014; 270: 27–39.
- Machado AR, de Assis LM, Machado MIR, de Souza-Soares LA. Importance of lecithin for encapsulation processes. Afr J Food Sci. 2014; 8: 176-183.
- Gobley TN. “Recherches Chimiques sur les Oeufs de Carpe,” J Pharm Chim. 1850; 17: 401-430 and 18: 107-119.
- Anwar MJ, Pillai KK, Samad A, Vohora D. Effect of escitalopram on cardiomyopathy-induced anxiety in mice. Human and Experimental Toxicology. 2013; 32: 632–639.
- Budai L, Kaszas N, Grof P, Lenti K, Maghami K, Antal I, et al. Liposomes for Topical Use: A Physico-Chemical Comparison of Vesicles Prepared from Egg or Soy Lecithin. Sci Pharm. 2013; 81: 1151–1166.
- Mertins O. Dissertacao de Mestrado; Estudo fisico-quimicos e estruturais de lipossomas compositos de fosfatidilcolina e quitosana. Universidade Federal do Rio Grande do sul, Brasil. 2004.
- Zambeli R, Moreira S. Definicao de lecitina. Engenharia de Alimentos. Universidade Federal doCeara, Brasil. 2009.
- Knuiman J, Beynen A, Katan M. Lecithin intake and serum cholesterol. Am J Clin Nutr. 1989; 49: 266 – 268.
- Orthoefer F. Lecithin and health. 1st edition. Illinois: Vital Health Publishing. 1998; 83.
- Oyewole AL, Owoyele BV. Neurobehavioural effects of exposure of wistar rats to smoke from traditional Carica papaya (pawpaw) leaves Int J of Genuine Traditional Medicine - TANG. 2012; 2: 36.
- Drent M, Cobben NAM, Henderson RF, Wouters EFM, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996; 9: 1736–1742.
- Lott JA, Nemensanszky E. Lactate dehydrogenase. In: Lott JA, Wolf PL, editors. Clinical Enzymology, a Caseoriented Approach. 1987; 213–244.
- Moss DW, Henderson AR. Enzymes. In: Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 2nd edition. Philadelphia, Saunders Co. 1986; 735-896.
- Jeng W, Loniewska WM, Wells PG. Brain Glucose-6-phosphate Dehydrogenase Protects against Endogenous Oxidative DNA Damage and Neurodegeneration in Aged Mice. ACS Chem Neurosci. 2013; 4: 1123-1132.
- Martini G, Ursini MV. A new lease of life for an old enzyme. Bioessays. 1996; 18: 631–637.
- Luzzatto L, Mehta A, Vulliamy T. Glucose-6-phosphate dehydrogenase (Chapter 179), in The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, editors. 8th edn., McGraw-Hill, New York. 2001; 4517-4553.
- Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the ?old? and update of the new mutations. Blood Cells Mol Dis. 2012; 48: 154-165.
- Tabatabaei SM, Khorashad AS, Sakeni M, Raeisi A, Metanat Z. Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency in southeast Iran: implications for malaria elimination. Infect Dev Ctries. 2015; 9: 289-297.
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008; 371: 64-74.
- Haller H, Cramer H, Lauche R, Gass F, Dobos GJ. The prevalence and burden of sub-threshold generalized anxiety disorder: a systemic review. BMC Psychiatry. 2014; 14: 128.
- Zhang T, Qu H, Li X, Zhao B, Zhou J, Li Q, Sun M. Transmembrane delivery and biological effect of human growth hormone via a phage displayed peptide in vivo and in vitro. J Pharm Sci. 2010; 99: 4880-4891.
- Rhoads DE, Huggler AL, Rhoads LJ. Acute and adaptive motor responses to caffeine in adolescent and adult rats. Pharmacol Biochem Behav. 2011; 99: 81-86.
- Muthappa NA, Gupta S, Yengkokpam S, Debnath D, Kumar N, Pal AK, et al. Lipotropes promote immunobiochemical plasticity and protect fish against low-dose pesticide-induced oxidative stress. Cell Stress Chaperones. 2014; 19: 61-81.
- Lu PZ, Lai CY, Chan WH. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci. 2008; 9: 698–718.
- Bavari M, Tabandeh MR, Varzi HN, Bahramzadeh S. Neuroprotective, antiapoptotic and antioxidant effects of L-carnitine against caffeine-induced neurotoxicity in SH-SY5Y neuroblastoma cell line. Drug Chem Toxicol. 2015; 1: 1–10.
- Berkowitz BA, Spector S. The effect of caffeine and theophylline on the disposition of brain serotonin in the rat. Eur J Pharmacol. 1971; 16: 322-325.
- Elyassi AR, Rowshan HH. Perioperative Management of the Glucose-6-Phosphate Dehydrogenase Deficient Patient: A Review of Literature. Anesth Prog. 2009; 56: 86-91.
- Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008; 326: 369-379.
- Borochov H, Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc NatI Acad Sci. 1976; 73: 4526-4530.
- Heron DS, Shinitzky M, Hershkowitz M, et al. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc NatI Acad Sci. 1980; 77: 7463-7467.
- Hughes RN, Hancock NJ. Strain-dependent effects of acute caffeine on anxiety-related behavior in PVG/c, Long–Evans andWistar rats. Pharmacology, Biochemistry and Behavior. 2016; 140: 51–61.
- Cubero J, Otalora BB, Bravo R, Sánchez CL, Franco L, Uguz AC, et al. Distribution of 5-HT receptors in the mammalian brain. Trends Cell Mol Biol. 2011; 6: 41–46.
- Nowicki M, Tran S, Muraleetharan A, Markovic S, Gerlai R. Serotonin antagonists induce anxiolytic and anxiogenic-like behavior in zebrafish in a receptor-subtype dependent manner. Pharmacol Biochem Behav. 2014; 126: 170-180.
- Eddie LG, Odette HG, Collinsworth MN, Garnovskaya TN, Tahir S, Venugopala B, et al. 5-HT2A receptors stimulate mitogen-activated protein kinase via H2O2 generation in rat renal mesangial cells. Am J Physiol Renal Physiol. 2000; 278: 650–658.
- Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. The FASEB Journal. 2005; 19: 641-643.