Case Report
Austin J Dermatolog. 2014;1(4): 1016.
A Case of Bullous Congenital Ichthyosiform Erythroderma, a Rare Pediatric Genodermatosis, in a Newborn
Millsop JW1*, Sivamani RK1, Lee DC2, Konia T1,3, Subramanian N2 and Fung MA1,3
1Department of Dermatology, University of California, USA
2Department of Pediatrics, University of California, USA
3Department of Pathology, University of California, USA
*Corresponding author: Millsop JM, Department of Dermatology, University of California, Davis, 3301 C Street, Suite 1400, Sacramento, CA 95816, USA
Received: July 10, 2014; Accepted: August 08, 2014; Published: August 13, 2014
Abstract
Bullous congenital ichthyosiform erythroderma is a rare autosomal dominant genodermatosis that typically presents in newborn infants. Mutations primarily of keratin 1 or keratin 10 causes, defective keratinization, leading to skin fragility, blistering, and hyperkeratosis. This condition can be difficult to distinguish from other exfoliative and bullous conditions, including staphylococcal scalded skin syndrome. We report a case of bullous congenital ichthyosiform erythroderma in a 12-hour-old infant and discuss the approach to management of the disease as well as the differential diagnoses.
Keywords: Bullous congenital ichthyosiform erythroderma; Epidermolytic hyperkeratosis; Ichthyosis; Blisters; Newborn
Abbreviations
BCIE: Bullous Congenital Ichthyosiform Erythroderma; ICU: Intensive Care Unit
Introduction
Bullous congenital ichthyosiform erythroderma (BCIE), also known as epidermolytic hyperkeratosis, is a rare autosomal dominant skin condition with a prevalence of 1 case per 200,000 to 300,000 individuals [1]. Up to 50 percent of cases can occur as sporadic mutations. BCIE is caused by mutations in keratin 1 or keratin 10, which are involved in suprabasilar keratinocyte differentiation [2- 6]. Mutations in keratin proteins lead to defective keratin formation and disruption of the keratin cytoskeleton. Furthermore, keratin mutations result in abnormal keratin filament clumping. These defects in the keratin protein network result in subsequent skin cell collapse and skin fragility. This clinically manifests as blistering, hyperkeratosis, and hyper proliferation.
Onset of BCIE typically occurs in newborns. At birth, patients present with generalized erythroderma, characterized by erythema and scaling, with or without edema over 90% of the skin [7]. Erosions, blisters, and peeling are typically present [7]. Although the blistering and erythema often improve over time, hyper keratotic scale becomes more prominent, most commonly over the neck, hands, feet, and joints. Other affected areas include the scalp and the infragluteal folds. An ichthyotic skin disorder persists throughout the lifetime of the patient.
BCIE can be difficult to distinguish from exfoliative skin diseases and bullous conditions. Herein, we present a case of BCIE in an infant, describe how the final diagnosis was obtained, and discuss management and treatment strategies for patients with BCIE.
Case Presentation
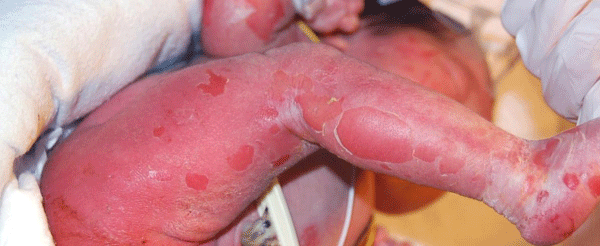
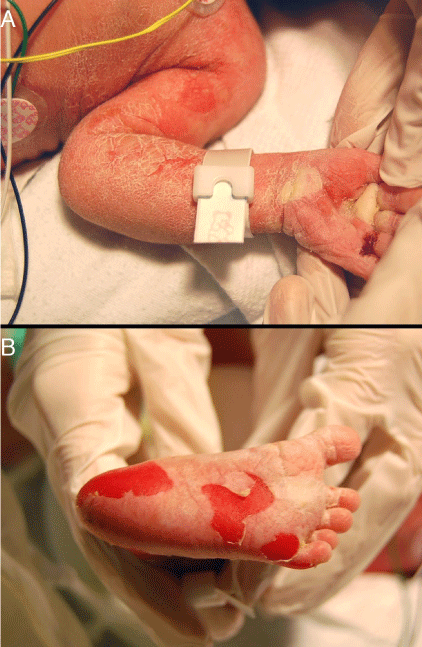
A 12-hour-old infant female presented with multiple erosions to the inpatient neonatal intensive care unit (NICU) at the University of California, Davis Medical Center after transferring from an outside facility. She was born by caesarean section at 36 weeks at the outside facility and was reported to be feeding well prior to her transfer. Blisters and erosions were noted at birth, at which point, the patient was transferred and she had been empirically started on vancomycin and gentamicin. There was no known history of bullous or ichthyosiform disease in her immediate family including two siblings; the extended family history was uncertain. Physical exam revealed an afebrile newborn with superficial erosions over the entire body, especially on the lower extremities and the groin (Figure 1). She had multiple bullae the left palm (Figures 2). These bullae and erosions were notably present on sites exposed to more friction such as the buttocks, thighs, posterior calves, palms, and soles. She was erythroderma with a fine white scale over the entire body. Her blood and wound cultures from the erosions were negative. Her blood chemistry and blood cell counts were within normal limits.
Figure 1: Diffuse white scaling was noted and the baby was erythrodermic. Multiple erosions were noted.
Figure 2: A) Two bullae were noted on the left palm. B) Multiple erosions were noted on the soles of the feet.
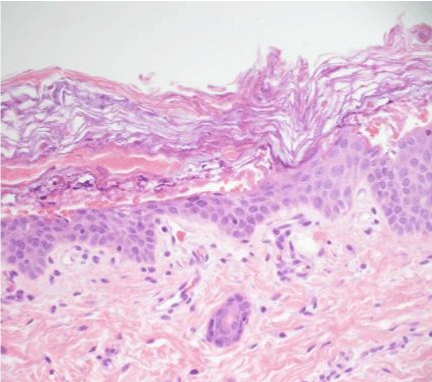
Based on her clinical presentation of generalized blistering, the differential diagnosis was wide including BCIE, ichthyosis bullosa of Siemens, staphylococcal scalded skin syndrome, bullous syphilis, bullous candidiasis, epidermolysis bullosa, and Stevens - Johnson syndrome. The fine white scale was most suggestive of an ichthyosiform disorder. Frozen section and formalin-fixed biopsies revealed hyperkeratosis associated with marked hyper granulose, variously sized and shaped prominent keratohyaline granules, eosinophilic globules, and vacuolated, feathery appearance of upper spinous cells (Figure 3). Genetic testing revealed a heterozygous E489K mutation on the keratin 1 gene, confirming the diagnosis of BCIE.
Figure 3: Epidermolytic hyperkeratosis. Hyperkeratosis associated with hypergranulosis, coarse keratohyaline granules, eosinophilic globules, and a vacuolated, feathery appearance of the upper spinous cells.
The infant was treated with topical emollients to the entire body, and her erosions healed with minimal scarring. The parents apply moisturizers daily, and the baby has remained free of secondary infections 15 months later. Although not present at birth, the infant has now developed hyperkeratosis skin of flexures of the arms and the legs, and the mother applies urea cream to reduce hyperkeratosis and prevent fissuring.
Discussion
BCIE is a congenital form of ichthyosis that causes disturbance of the epithelial barrier, which may lead to severe infection and sepsis if not identified and controlled. Therefore, for a pediatric patient with possible BCIE, it is important for clinicians to consider this diagnosis in any newborn born with multiple bullae and generalized white scaling. The genetic defects are typically found in keratins 1 or 10 with acral bullae forming in those with keratin 1 defects [1], as was present in the case described here. Keratins 1 and 10 are significant structural proteins that are found in the granular and suprabasilar epidermal layers. Although BCIE is associated with many different mutations, the main alterations attributed to the disease are point mutations in the 1A and 2B domains of the alpha helix that cause an amino acid change [8,9].
In the setting of newborns with blisters, it is important to rule out staphylococcal scalded skin syndrome (SSSS), a toxin-mediated skin condition that presents with blistering, widespread erythema, and exfoliation. However, this patient had no signs of infection with normal vital signs, normal interaction with the mother, and negative blood cultures. The fine white scale served as an important clue as this supported an ichthyotic etiology over SSSS. Furthermore, the erosions and erythema of SSSS are usually focused on the flexural surfaces whereas the erosions were in friction dependent areas in the case presented. Histologically, BCIE shows epidermolytic hyperkeratosis, characterized by acanthuses, prominent ("coarse") keratohyaline granules, eosinophilic globules and perinuclear bands (representing clumps of defective keratin) [10,11], perinuclear vacuoles, and hyperkeratosis with ballooning degeneration of the granular and spinous layers of the epidermis [1]. These same histopathologic features are generally confined to the superficial portions of the epidermis in ichthyosis bullosa of Siemens and to acral skin in epidermolysis palm plantar keratoderma. In contrast, staphylococcal scalded skin syndrome is characterized by a non-inflammatory, non-epidermolytic intraepidermal acantholytic cleavage plane restricted to the granular cell layer [12].
BCIE can be passed in an autosomal dominant fashion or can develop sporadically [7]. In some cases, the mother can have a mild presentation of disease due to somatic mosaicism [13], but there was no evidence of localized disease in either of the patient's parents. Genetic prenatal counseling should be offered to the family for future pregnancies, and a prenatal diagnosis can be made through fetal skin biopsy, amniocentesis, and chorionic villus sampling [14].
Initial treatment early in disease is targeted towards symptomatic relief and management of the secondary complications of the erosions. These complications include electrolyte imbalance, dehydration, infection, and sepsis. Erosions need to be managed meticulously with barrier protection and gentle handling of the skin to minimize the development of secondary infection. Bacterial overgrowth, particularly from Staphylococcus aureus, and an odor can develop as a result of scale accumulation, which may be controlled with chlorhexidine or antibacterial cleansers. Later in disease, emollients as well as topical and systemic retinoids can be considered. Topical emollients are considered mainstay therapy, as well as creams or ointments that possess keratolytic properties to reduce the hyperkeratosis scale that develops in these patients. Examples include urea, alpha-hydroxy acids [15], lactic acid, and glycerin, although lactic acidosis is a concern with topical lactic acid in infants and small children [16]. Topical retinoid, such as retinoic or tazarotene, may be useful and systemic retinoids may be needed for more severe cases [17-19]. Acitretin is an effective systemic option for BCIE with typical treatment doses ranging from 5 mg every other day (in a 10-year-old boy with no weight based dosing reported) to 25 mg daily [17-19]. Isotretinoin has also been shown to be effective [20], as demonstrated in a multicenter study composed of twenty-two patients showing improvement in 90% of those treated [21]. Treatment doses were typically at 2 mg/kg with a maximum of 4 mg/ kg. Topical and systemic retinoids appear to be more effective in those with defects in keratin 10 rather than keratin 1 although isotretinoin was not included as a treatment in this study [19].
Bullous congenital ichthyosiform erythroderma is rare and has a challenging differential diagnosis. Nevertheless, it is important for clinicians to identify the disease early in order to reduce morbidity and mortality as evidenced by this case.
References
- DiGiovanna JJ, Bale SJ. Clinical heterogeneity in epidermolytic hyperkeratosis. Arch Dermatol. 1994; 130: 1026-1035.
- Cheng J, Syder AJ, Yu QC, Letai A, Paller AS, Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992; 70: 811-819.
- Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, et al. A leucine-proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992; 70: 821-828.
- Rothnagel JA, Dominey AM, Dempsey LD, Longley MA, Greenhalgh DA, Gagne TA, et al. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992; 257: 1128-1130.
- Yang JM, Chipev CC, DiGiovanna JJ, Bale SJ, Marekov LN, Steinert PM, et al. Mutations in the H1 and 1A domains in the keratin 1 gene in epidermolytic hyperkeratosis. J Invest Dermatol. 1994; 102: 17-23.
- Chipev CC, Yang JM, DiGiovanna JJ, Steinert PM, Marekov L, Compton JG, et al. Preferential sites in keratin 10 that are mutated in epidermolytic hyperkeratosis. Am J Hum Genet. 1994; 54: 179-190.
- Kwak J, Maverakis E. Epidermolytic hyperkeratosis. Dermatol Online J. 2006; 12: 6.
- DiGiovanna JJ, Bale SJ. Epidermolytic hyperkeratosis: applied molecular genetics. J Invest Dermatol. 1994; 102: 390-394.
- Virtanen M, Smith SK, Gedde-Dahl T Jr, Vahlquist A, Bowden PE. Splice site and deletion mutations in keratin (KRT1 and KRT10) genes: unusual phenotypic alterations in Scandinavian patients with epidermolytic hyperkeratosis. J Invest Dermatol. 2003; 121: 1013-1020.
- E Shabrawi-Caelen L, McCalmont TH. Perinuclear eosinophilic bands: a clue to keratin gene mutation. J Cutan Pathol. 2010; 37: 718-719.
- Bergman R, Khamaysi Z, Sprecher E. A unique pattern of dyskeratosis characterizes epidermolytic hyperkeratosis and epidermolytic palmoplantar keratoderma. Am J Dermatopathol. 2008; 30: 101-105.
- Patel GK, Finlay AY. Staphylococcal scalded skin syndrome: diagnosis and management. Am J Clin Dermatol. 2003; 4: 165-175.
- Nomura K, Umeki K, Hatayama I, Kuronuma T. Phenotypic heterogeneity in bullous congenital ichthyosiform erythroderma: possible somatic mosaicism for keratin gene mutation in the mildly affected mother of the proband. Arch Dermatol. 2001; 137: 1192-1195.
- Rothnagel JA, Lin MT, Longley MA, Holder RA, Hazen PG, Levy ML, et al. Prenatal diagnosis for keratin mutations to exclude transmission of epidermolytic hyperkeratosis. Prenat Diagn. 1998; 18: 826-830.
- Kempers S, Katz HI, Wildnauer R, Green B. An evaluation of the effect of an alpha hydroxy acid-blend skin cream in the cosmetic improvement of symptoms of moderate to severe xerosis, epidermolytic hyperkeratosis, and ichthyosis. Cutis. 1998; 61: 347-350.
- Ramírez ME, Youseef WF, Romero RG, Martínez JM, González-Enseñat MA, Vilaplana XS, et al. Acute percutaneous lactic acid poisoning in a child. Pediatr Dermatol. 2006; 23: 282-285.
- Kocaturk E, Zemheri E, Kavala M, Aktas S, Sarigul S, Sudogan S. Two cases of linear epidermolytic hyperkeratosis: therapeutic challenge with acitretin. Eur J Dermatol. 2010; 20: 404-405.
- Nassif PW, Nakandakari S, Fogagnolo L, Contin LA, Alves CJ. Epidermolytic hyperkeratosis: a follow-up of 23 years of use of systemic retinoids. An Bras Dermatol. 2011; 86: S72-75.
- Virtanen M, Gedde-Dahl T Jr, Mörk NJ, Leigh I, Bowden PE, Vahlquist A. Phenotypic/genotypic correlations in patients with epidermolytic hyperkeratosis and the effects of retinoid therapy on keratin expression. Acta Derm Venereol. 2001; 81: 163-170.
- Achar A, Naskar B, Laha R, Ray S. Epidcermolytic hyperkeratosis: a case report. J Indian Med Assoc. 2009; 107: 171-172.
- Baden HP, Buxman MM, Weinstein GD, Yoder FW. Treatment of ichthyosis with isotretinoin. J Am Acad Dermatol. 1982; 6: 716-720.