
Case Report
Austin J Dermatolog. 2023; 10(1): 1103.
Improvement of Atopic Keratoconjunctivitis during Treatment with Upadacitinib for Atopic Dermatitis
Ghiglioni DG1*, Cozzi L2, Pigazzi C2, Bruschi G2, Osnaghi S3, Colonna C4, Mapelli C3, Galimberti D3, Marchisio PG5 and Ferrucci SM6
1Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, SC Pediatria Pneumoinfettivologia, Italy
2University of Milan, Italy
3Fondazione IRCCS Ca’ Granda Ospedale MaggiorePoliclinico di Milano, SC Oculistica, Italy
4Fondazione IRCCS Ca’ Granda Ospedale MaggiorePoliclinico di Milano, Pediatric Dermatology Unit, Italy
5Department of Pathophysiology and Transplantation,University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, SC Pediatria Pneumoinfettivologia, Italy
6Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, SC dermatologia, Italy
*Corresponding author: Ghiglioni DGFondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, SC Pediatria Pneumoinfettivologia, University of Milan, Italy
Received: January 26, 2023; Accepted: February 22, 2023; Published: February 28, 2023
Abstract
Background: Upadacitinib is a small oral molecule that selectively inhibits Janus Associated Kinase (in particular JAK1) approved by FDA in 2022 for children (12 years and older) with refractory, moderate to severe atopic dermatitis. Nowadays there is a gap in knowledge about real world data (and comparison with Dupilumab) after very promising trials.
Methods: Clinical images, disease scores and quality of life scales were collected in the first report about upadacitinib efficacy in a young AKC patient, in which Dupilumab did not allow the expected improvement in both the skin and the eyes.
Results: Ocular symptoms and skin lesions sensibly improved after upadacitinib therapy (week 0: EASI 30, NRS ITCH 10, NRS SLEEP 8, DLQI 15, POEM 26 and week 52: EASI 0, NRS ITCH 2, NRS SLEEP 0, DLQI 1, POEM 1). Ocular symptoms (itching, photophobia and redness) improved, periocular skin healed. Tarsal papillary hypertrophy faced complete resolution replaced with scar tissue, corneal neovascularization resolved.
Conclusions: Upadacitinib showed great clinical efficacy on DA and AKC and an even greater effect on quality of life. Upadacitinib must be taken into account in a patient with Atopic Keratoconjunctivitis (AKC) considering that major adverse reaction of Dupilumab is conjunctivitis.
Keywords: Upadacitinib; Atopic keratoconjunctivitis; Atopic dermatitis; Ocular allergy; Biologics
Introduction
Atopic Keratoconjuntivitis (AKC) is a chronic allergic ocular disease that damages ocular surface and, if not treated properly, leads to corneal scarring and vision loss. The exact prevalence is unknown, about 4% of patients with ocular allergy are affected in big cohorts [1]. Atopic Dermatitis (AD) is a common chronic inflammatory skin disease with an increasingly higher prevalence in adults, in Italy it’s estimated at 8.1% [2]. Upadacitinib is a small oral molecule that selectively inhibits Janus Associated Kinase (in particular JAK1) approved by Food and Drug (FDA) in 2022 for adult and children (12 years and older) with refractory, moderate to severe atopic dermatitis.
Case Presentation
We describe the case of a young Caucasian man whose AKC symptoms sensibly improved after upadacitinib was prescribed for his AD. The patient suffered from AD since his first year of life. During infancy he developed seasonal rhino-conjunctivitis and asthma (sensitization to graminaceae was documented). Nasal and respiratory symptoms improved with puberty.
The conjunctivitis progressively became persistent during summertime and the presence of a significant upper tarsal papillary hypertrophy led to a diagnosis of vernal keratoconjunctivitis at the age of eight. Two years later, as prolonged therapy with corticosteroids was needed, topical cyclosporine 1% in galenic preparation (3 applications/day) was introduced as a corticosteroid-sparing agent. Symptoms then became perennial and lower tarsal conjunctivae became involved: a reclassification as AKC was made at the age of fifteen and, as tarsal papillary hypertrophy persisted, a switch to topical tacrolimus 0, 1% in galenic preparation (3 applications/day) was decided.In the meanwhile, his AD showed a fluctuating trend. The persistency of moderate/severe dermatitis led to the introduction of topical calcineurin inhibitors (tacrolimus ointment 0, 03%, later replaced by pimecrolimus cream 1%) since the age of twelve. The skin lesions slightly improved at the age of fourteen while wheat was temporarily excluded from his diet (allergen-specific IgE were found positive).
Both the skin lesions and eye lesions where poorly controlled despite the ongoing therapies (Figure 1). Oral cyclosporine (5 mg/kg/day) was hence started at the age of sixteen. Tacrolimus eye drops were continued. A significant improvement of cutaneous and, partially, ocular symptoms was observed. However, any attempt of cyclosporine dose tapering was correlated with relapses. Therefore, at the age of nineteen, subcutaneous dupilumab (600 mg once, then 300 mg twice monthly) was introduced allowing a better control of the dermatitis while gradually reducing cyclosporine until its withdrawal seven months later. Various flare-ups of the AKC occurred, presumably dupilumab-related, and topical corticosteroids as rescue therapy were required. In consideration of the dupilumab-related worsening of keratoconjunctivitis and a poor dermatitis control, dupilumab was discontinued after 1, 5 years of therapy (Figure 1). Six months later, given the persistent and severe clinical conditions, oral therapy with upadacitinib (30 mg/day) was started at the age of twenty-one. At the start of the therapy the score were: EASI 30, NRS itch 10, NRS sleep disturbance 0, DQLI 15). After only one month EASI 0, NRS itch 0, NRS sleep disturbance 0, DQLI 0 (see Table 1 and Figure 2 for systematic and graphical representation of scores). A year after its introduction, upadacitinib led to a good control of AKC and AD (Figure 1). Seven months after the beginning of the therapy, a reintroduction of wheat into the diet was decided and it’s currently well-tolerated. Follow-up showed a mild hyperCKemia (x1,5 upper normal level) and newly diagnosed acne.After ubadacitinib introduction, tacrolimus collyrium was replaced with cyclosporine collyrium 1% (twice daily) that was stopped in June 2022 following a further clinical improvement. The actual therapy includes upadacitinib, skin emollients and artificial tears with optimal control of disease.
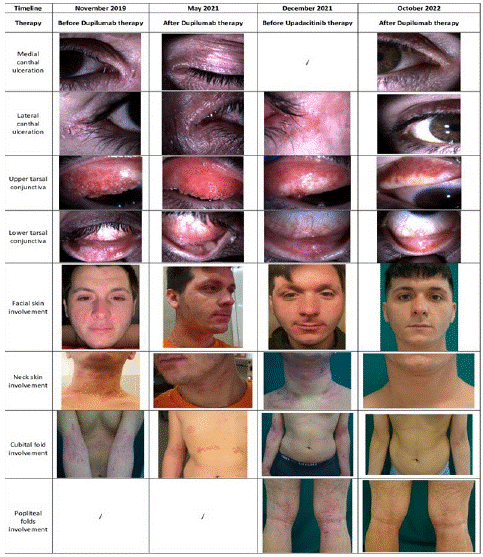
Figure 1: Ocular and skin involvement in relation to biologic therapy. Before biologics, mild palpebral eczema was associated with medial and lateral canthal ulceration. The eyelid margins ware thickened and irregular. The lower lids presented Meibomian gland dysfunction, squamous metaplasia and symblepharon. Fibrosis and scarring of the upper tarsal conjunctiva were observed. Corneal neovascularization occurred. Dupilumab therapy caused worsening of keratoconjunctivitis and a poor dermatitis control. After Upadacitinib therapy skin itching has improved. Slightly lichenified areas persist, being mostly localized over the antecubital fossa and neck. Ocular symptoms (itching, photophobia and redness) have also improved. Periocular skin has healed. Squamous metaplasia and symblepharon persist. Tarsal papillary hypertrophy has faced complete resolution and has been replaced with scar tissue. Corneal neovascularization resolved.
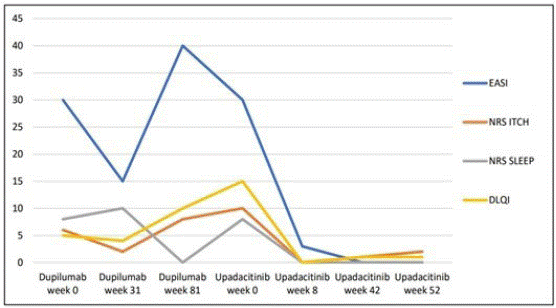
Figure 2: The picture shows the trend of the scores in the different stages of the disease. After an initial improvement with dupilumab, the DA worsened and dupilumab was discontinued. A rapid and significant improvement was observed after the introduction of upadacitinib. Since then, the therapy was continued and the disease is currently well controlled.
Dupilumab
week 0
13/11/2019Dupilumab
week 31
16/06/2020Dupilumab
week 81
31/05/2021Upadacitinib
week 0
16/12/2021Upadacitinib
week 8
08/02/2022Upadacitinib
week 42
04/10/2022Upadacitinib
week 52
20/12/2022EASI
30
15
40
30
3
0
0
NRS ITCH
6
2
8
10
0
1
2
NRS SLEEP
8
10
0
8
0
0
0
DLQI
5
4
10
15
0
1
1
POEM
11
26
3
1
1
HADS-D
8
3
7
6
6
6
HADS-A
5
1
7
4
6
2
Table 1: At follow-up visits, various scales were used to assess DA severity and the quality of life. Here are reported the scores recorded before and during dupilumab therapy and after the introduction of upadacitinib. EASI (Eczema Area and Severity Index); NRS ITCH (Itch Numeric Rating Scale), NRS SLEEP (Sleep Quality Numeric Rating Scale), DLQI (Dermatology Life Quality Index), POEM (Patient Oriented Eczema Measure), HADS (Hospital Anxiety and Depression Scale).
Discussion
An extensive research was performed on several bibliographic biomedical databases (Pubmed, SCOPUS, EMBASE, GOOGLE SCHOLAR, and COCHRANE LIBRARY) in September 2022. This appeared to be the first report about upadacitinib efficacy in a young Caucasian adult with AKC. The efficacy and safety of upadacitinib in AD have been described in clinical trials, but real-world data are lacking given the short time since its approval for AD, in January 2022 [3]. A research with key words “upadacitinib” and “conjunctivitis” was performed: a single case of upadacitinib-related conjunctivitis improvement was described by Hayama [4]. However, it was a dupilumab-related conjunctivitis in a patient with atopic dermatitis with no previous ocular involvement.In our case upadacitinib led to a significant clinical improvement of skin and ocular disease. An even greater effect in quality of life (as assessed by QOL scale) was reported by the patient, who described the new therapy as “life-changing”. An improved wheat tolerance was observed after upadacitinib was started. There’s no evidence about its possible effect on tolerance thresholds, but a few trials document the role of biological therapies (dupilumab and omalizumab) as adjuvants for oral desensitization. Several inflammatory cytokines are involved in the pathogenesis of AD and many pathways are not yet clearly understood. The JAK1 signal pathway, which upadacitinib selectively inhibits, stops the signaling of a wide range of proinflammatory mediators: IL-4 and IL-13 (that cause epidermal dysfunction in AD), INF-gamma (lesion chronicity in AD), IL-22 (involved in epidermal hyperproliferation), IL-31 (involved in itch neuron stimulation), thymic stromal lymphopoietin (that triggers TH2 cell differentiation) [5]. The patient was previously treated with dupilumab, a subcutaneous monoclonal antibody directed against subunit alpha of interleukin 4 receptor approved since June 2022 by Food and Drug Administration for patients with severe AD since 6 months of age [3]. The major adverse reaction of dupilumab is conjunctivitis [6]. Conjunctivitis was observed in 8.6-22.1% of patients in clinical trials, but it’s even more prevalent (up to 62%) in real-word reports [7]. However, in most cases it seemed to be mild and transient [8]. The possibility of a severe conjunctivitis must be kept into account in AD patients suffering also from ocular diseases, as AKC. In different reports upadacitinib showed a quicker and more effective activity in moderate to severe AD than dupilumab [6]. The simultaneous inhibition of several cytokines contributes to the efficacy and rapid effects of this drug [5]. It is reported that dupilumab can worsen facial and periorbital eczema. Upadacitinib would therefore be a better option for AD with facial involvement. A case series reported improvement in facial symptoms after switching from dupilumab to upadacitinib [6]. The JAK inhibitor moreover showed a beneficial impact on inflammatory comorbidities, such as Crohn’s disease and alopecia areata [9]. These are encouraging points about a possible causal (and not casual) positive effect also on atopic keratoconjuntivitis.Nowadays anti-JAK1 is approved for psoriasis, active ankylosing spondylitis, alopecia and vitiligo. Compared with other JAK inhibitors (tofacitinib, baricitinib, ruxolitinib), upadacitinib acts selectively on JAK1 and thus provokes a lower range of adverse reactions. Common side effects (>10%) are superior airways infection and acne [10]. Increase of creatine phosphokinase, transaminases, cholesterol and leucopenia or anemia can also occur. The limits of this report include a short follow-up time, the previous use of other drugs and the lack of other cases.
Conclusions
After a year of follow-up, even if considering the previous use of other drugs, upadacitinib showed great clinical efficacy on DA and AKC and an even greater effect on quality of life. Upadacitinib must be taken into account in a patient with severe Atopic Keratoconjunctivitis (AKC) considering that the major adverse reaction of Dupilumab is conjunctivitis. Further reports and studies are needed to better understand the efficacy of upadacitinib in AD <12 years old and in AKC. However, upadacitinib efficacy on AD, its properties on other dis-immune diseases associated with AD and its easy handling (oral administration and room temperature storage possibility) made this JAK1 inhibitor a new extremely promising option.
References
- Uchio E, Kimura R, Migita H, Kozawa M, Kadonosono K. Demographic aspects of allergic ocular diseases and evaluation of new criteria for clinical assessment of ocular allergy. Graefe’s Arch Clin Exp Ophthalmol. 2008; 246: 291-296.
- Chiricozzi A, Gori N, Narcisi A, Balato A, Gambardella A, et al. Effectiveness and Safety of Upadacitinib in the Treatment of Moderate-Severe Atopic Dermatitis: A Multicentric, Prospective, Real-World, Cohort Study. Drugs R D. 2022; 22: 245-252.
- Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021; 397: 2151-2168.
- Hayama K, Fujita H. Improvement of dupilumab-associated conjunctivitis after switching to upadacitinib in a patient with atopic dermatitis. Dermatol Ther. 2022; 35: 35-36.
- Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, Bruin-Weller MD, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatology. 2021; 157: 1047-1055.
- Licata G, Gambardella A, Tancredi V, Calabrese G, Rosa AD, et al. Face atopic dermatitis resistant to dupilumab: a case series of three patients successfully treated with upadacitinib. J Eur Acad Dermatology Venereol. 2022; 36: e150-e152.
- Kamata M, Tada Y. A Literature Review of Real-World Effectiveness and Safety of Dupilumab for Atopic Dermatitis. JID Innov. 2021; 1: 100042.
- Guex-Crosier Y, Di-Lucca J, Häusermann P, Laffitte E, Saulite L, et al. Management of dupilumab-associated ocular surface diseases in atopic dermatitis patients. Swiss Med Wkly. 2021; 151: 1-10.
- Dal Bello G, Schena D, Girolomoni G. Upadacitinib in patients with resistant atopic dermatitis: a retrospective case-series. J Eur Acad Dermatology Venereol. 2022; 36: 1499-1500.
- Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021; 397: 2169-2181.