
Research Article
Austin J Dermatolog. 2023; 10(1): 1107.
The Association of Propranolol with 2-Deoxy-D-Glucose Reduces the Metabolism and the Proliferation of Cutaneous Squamous Carcinoma A431 Cell Line
Carolina V De Almeida1; Marianna Buscemi1, Aida Cavallo1, Matteo Lulli2, Ilenia Foffa1, Tamer Al Kayal1, Giorgio Soldani1, Paola Losi1*
¹Institute of Clinical Physiology, CNR, Massa, Italy
²Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, Università degli Studi di Firenze, Italy
*Corresponding author: Paola Losi Institute of Clinical Physiology, CNR, Via Aurelia sud, 54100, Massa, Italy. Email: paola.losi@ifc.cnr.it
Received: June 12, 2023 Accepted: July 12, 2023 Published: July 19, 2023
Abstract
Non-selective β-blocking (±)-Propranolol Hydrochloride was demonstrated to improve the progression-free survival of oncological patients. Since the expression of β-adrenoceptors in the epidermal squamous cell carcinoma was described, we hypothesized that the topical application of a β- adrenoceptors-blockers over the tumor lesion may decrease its extension before the surgical excision, becoming an adjuvant therapy against cutaneous squamous cell carcinoma. However, it is known that β-AR-blocker anti-cancer activity as a single agent is limited. Hence, we suggested that the combination of propranolol with the glucose analog 2-Deoxy-D-glucose could improve its antiproliferative effect through the induction of metabolic stress.
Propranolol and 2-Deoxy-D-glucose effect on A431 squamous cell carcinoma and normal keratinocytes evaluating metabolic activity, proliferation and apoptosis through MTT, immunofluorescence Ki-67 and AnnexinV assays, cell cycle analysis and migration assay.
Our results demonstrated that the addition of 2-Deoxy-D-glucose low dose to propranolol improve its effect on reduction of A431 cells metabolism and proliferation, similar effect was observed on HaCaT viability and mobility. However, the HaCaT migration ability is not completely compromised.
The combination of propranolol with a low dose of 2-Deoxy-D-glucose could be a promising treatment to be topically applied avoiding systemic adverse effects in patients with cutaneous squamous cell carcinoma.
Keywords: Propranolol hydrochloride; 2-Deoxy-d-glucose; Cutaneous squamous cell carcinoma; A431 cell line
Introduction
Cutaneous Squamous Cell Carcinoma (cSCC) is the second most frequent skin cancer in white ethnic populations worldwide [1,2] and even if most of the cases are easily cured by surgical removal, this cancer remains the cause of the majority of Non-Melanoma Skin Cancer (NMSC) deaths. This is due to “high-risk SCCs”, which are associated with significant metastasis, morbidity, and death [3,4]. Among the main cause of cSCC, DNA damage by Ultraviolet (UV) radiation exposure is the most common [5], since it causes the deregulation of important signaling pathways that are involved in the cell cycle, apoptosis, DNA repair and cell differentiation [6,7]. Other risk factors for cSCC promotion are immunosuppression [8], Human Papilloma Virus (HPV) infection [9,10], genetic disorders [11] and smoking [12]. Sporadically, cSCC can be also associated with non-healing wounds/scarring, or chronic lesions preceded by chronic inflammatory processes [13, 14].
Usually, in situ cSCC may be controlled by different interventions, including electrodessication and curettage, topical therapy, cryotherapy, and photodynamic therapy; however, since these treatments are not appropriate for invasive cSCC [15], surgical excision is usually indicated. The surgical procedure creates wounds that could be small, superficial, and amenable to primary closure, but often they can be large, deep, and extensive needing more complex closure and covering. Specifically scalp injuries, due to low elasticity, can be devastating and can require significantly more extensive surgeries, concerning both the number and complexity [16]. Consequently, we believe that an effective therapeutic strategy could be to control the extension of the lesion before its excision aspiring for less invasive surgery with less devastating wounds.
Multiple intracellular signal transduction pathways, involved in events such as cellular replication, inflammation, angiogenesis, apoptosis, cell motility and trafficking, activation of tumor-associated viruses, DNA damage repair, cellular immune response and epithelial–mesenchymal transition (reviewed by Coelho M et al) [17], are regulated through interactions of a- and β-Adrenoceptors (AR) and Catecholamine (CA) neurotransmitters [18]. Tumor cells may express β-AR, and the involvement of β-adrenergic signaling in the progression of malignant diseases has been increasingly [1] recognized [19-21]. The use of beta-blocker therapy can reduce the incidence of prostate cancer [22] and improve the prognosis of patients with breast [23] and hepatocellular [24] cancer. The expression of β-AR in the A431 cSCC human cells was described in 1987 by Kashles and Levitzki [25], which leads us to believe that cSCC proliferation may be controlled by using β-AR-blockers. Thus, we hypothesized that the topical application of a β-AR-blocker over the tumor lesion may decrease/restrain its extension before the surgical excision, becoming an adjuvant\therapy against cSCC. The topical application of β-AR-blocker, timolol [26,27] and propranolol [28], was already described on infantile hemangioma, with no collateral effects.
Recent evidence has shown that non-selective β-blocking (±)-Propranolol Hydrochloride, improved the progression-free survival of breast cancer patients [29], and reduced the risk of developing head and neck, prostate, esophagus, stomach, and colon cancers [30] It is known that the (±)-Propranolol Hydrochloride anti-cancer activity is due to its ability to inhibit the mitochondrial metabolism, which can increase the cell glycolytic activity resulting in elevated metabolism and switch towards aerobic glycolysis, which could stimulate the tumor progression and drug resistance. However, its anti-cancer activity as a single agent was demonstrated to be limited [31].
Hence, based on the well-known warburg effect, cancer cells boost glucose uptake and conversion into lactate in the presence of high oxygen tension, exploiting the aerobic glycolysis, we suggested the combination of propranolol with the glucose analog 2-Deoxy-D-glucose (2-DG) aiming to improve its antiproliferative effect. 2DG is a well-known antidiabetic drug, which by competition can inhibit glucose uptake, blocking the first critical step of glucose metabolism and mitochondrial respiration, inducing metabolic stress [32]. 2DG increases autophagy, a ubiquitous cellular catabolic process that under conditions of protracted stresses suppresses tumorigenesis [33]. 2DG treatment alone does not significantly induce cancer cell death, but it may use with specific agents or to exert a synergistic therapeutic action.
To confirm these hypotheses, we performed in vitro assays using the human A431 cSCC cell line and the human keratinocytes HaCaT cells. We demonstrated that the addition of 2DG to (±)-Propranolol Hydrochloride therapy can improve its effect on A431 cells metabolism and proliferation.
Materials and Methods
Propranolol Solution
(±)-Propranolol Hydrochloride (Sigma St. Louis, MO, USA) dilutions were made using 1:1 (v:v) Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 Ham (SF-DMEM: F12) (Sigma St. Louis) in a stock solution of 400μM, filtered with a 0.22μm (Millipore, Burlington, MA, USA). The solution was diluted freshly before each experiment to different concentrations as indicated in each assay. Corroborating with the results of Bustamante et al. 2019 [34] whom demonstrated that on melanocytes 200μM of propranolol has a cytotoxic effect, but not with 50μM, we started to observe effects only with concentrations over 100μM (data not shown), thus, we performed the experiments using concentrations from 100 to 300μM (100, 150, 200 and 300μM).
2DG Solution
2-Deoxy-D-glucose (Calbiochem, San Diego, CA, USA) dilutions were made using 1:1 (v:v) Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 Ham (SF-DMEM:F12) (Sigma St. Louis) in a stock solution of 5mM, filtered with a 0.22μm (Millipore). The solution was diluted freshly before each experiment to different concentrations as indicated in each assay. Based on the literature, we investigated the cytotoxic effect of 2DG in five different concentrations: 1, 2, 3, 4 and 5mM.
Cell Culture
The epidermal squamous cell carcinoma A431 (ATCC® CRL-1555™) (Cell Applications, San Diego, CA, USA) was cultivated on DMEM/Ham's F12 medium (Sigma St. Louis), added with 1x nonessential amino acids (Lonza™ BioWhittaker™. Basel, Switzerland), L-glutamine (2.5mM), gentamicin (1μL/mL) and FBS 10%. The medium was routinely changed every 3 days and at confluence, cells were subcultured (split ratio 1:5) by trypsinization (0.5% trypsin/0.02% EDTA). Human keratinocytes HaCat (Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna "Bruno Ubertini") were cultured in RPMI 1640 supplemented with 10% FBS, 2mM L-Glutamine, 100μg/mL streptomycin and 100U/mL penicillin.
Metabolic Viability – MTT Assay
To evaluate the effect of different doses of propranolol on cellular metabolic activity, we used the colorimetric assay with MTT [3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]. Briefly, cells (5x103 cells/well) were seeded into 96-well plates. After 24h of incubation, the medium was added with the specific doses of propranolol diluted in complete cell culture medium (150–350μM), or complete medium which was used as a reference. After 48h, MTT phosphate buffered solution (final concentration of 0.1mg/mL) was added to each well and cultures were incubated at 37°C for 3h. The supernatant was removed from the wells by slow aspiration and replaced with DMSO (100μl per plate) to solubilize the MTT tetrazolium dye. At the end of incubation time, the Optical Density (OD) was measured at 550nm wavelength using a microplate reader (Spectrafluor Plus; TECAN Austria GmbH, Grödig, Austria). Three replications were used for each analysis. The percentage of cell viability was calculated vs. the complete medium (assumed as 100%).
Cell proliferation Assay
Cells were seeded (5x103 cells/well) in a 96-wells plate and incubated for 24h. Then, we treated the cells with 200μM of propranolol (Prop), 0.5mM 2DG (2DG) or 200μM of propranolol and 0.5mM of 2DG (Prop+2DG). Control was made by adding fresh complete medium. Three replications were used for each analysis. Cells were detached and alive cells were manually counted in three times: before treatments, 24 and 48h after the treatments.
Immunofluorescence Ki-67
Cells were grown overnight on glass coverslips and then treated with 200μM of propranolol during 24h. Cells were washed twice with 1mL of cold Phosphate Buffered Saline (PBS), fixed for 20 min in 3.7% paraformaldehyde in PBS and permeabilized with 0.3% Triton X-100 in PBS for 5 min. Cells were incubated in the blocking buffer (5% FBS and 0.3% Triton X-100 in PBS) for 1 h at room temperature. Then, the cells were incubated overnight at 4°C with primary antibody Ki-67 (sc-23900) (Santa Cruz Biotechnology, Dallas, TX, USA) and successively for 1h with the anti-mouse Alexa Fluor 488 (Cat #A21121) (ThermoFisher, Waltham, MA, USA) at room temperature. After staining of the nuclei with Hoechst 33242 dye (Sigma St. Louis), the cells were dried, mounted onto glass slides with DPX Mountant for histology (Sigma St. Louis), and examined with confocal microscopy using a Nikon Eclipse TE2000-U (Nikon, Tokyo, Japan). A single composite image was obtained by the superimposition of 6 optical sections for each sample observed. The collected images were analyzed by Image J software. All the experiments were repeated three times.
Apoptosis Assay
Apoptosis was detected by flow cytometry by using (BV421)-Annexin-V and the nonvital dye 7-amino-actinomycin D (7AAD) double staining (BD Biosciences, Franklin Lakes, NJ, USA). A431 cells were inoculated into six-well plates with 5x105 cells/well and cultured for 24h. The growth of cells converged to approximately 70%. Cells were then treated with Prop, 2DG or Prop+2DG. After 24h of treatment, floating and adherent cells were collected and resuspended in binding buffer (BD Biosciences). (BV421)-Annexin V and 7AAD were added, the samples were incubated for 20 min in the dark at 4°C and analyzed by FACSCanto II and FlowJo software (BD Biosciences). Experiments were performed three times.
Cell Cycle Analysis
Flow cytometry analysis of DNA content was performed to assess the cell cycle phase distribution in control conditions (not treated) or after the treatments were added to logarithmically growing A431 cells. After 48h exposure, A431 cells were harvested by trypsinization, and a solution containing 50μg/mL propidium iodide (Sigma Aldrich), 0.1% w/v trisodium citrate and 0.1% NP40 was added. Samples were then incubated for 30min at 4°C in the dark and nuclei analysed with a FACSCanto II flow cytometer and FlowJo software (BD Biosciences) Experiments were performed three times.
Cell Migration Assay
The cells (20x104) were seeded into 24-well plates, cultured to confluence, and scratched by scraping with a 10μL pipette tip. Following PBS washes, cultures were treated with Prop, 2DG or Prop+2DG. Control wells received a serum-free medium or complete culture medium. At 20, 40 and 60h after scratching, digital images of cells were captured by a phase contrast microscope (Axiovert 25, Zeiss, Milan, Italy; O.M. 50X) equipped with a digital camera (EOS 1000D, Canon, Milano, Italy). Scratch closure was qualitatively analyzed with respect to time 0, by measuring the wound width/area change. Such method uses several metrics to quantify migration, including the percentage difference in the wound width at each time point.
Statistical Analysis
Differences in the experimental groups were assessed using analysis of variance (ANOVA). To avoid bias due to the variability between the experiments, the factor defining the different experimental groups was crossed with a second factor defining the different experiments (two-way ANOVA). P-values lower than 0.05 were considered statistically significant. Figures are representative of all experiments that were realized during the study.
Results
Combination of (±)-Propranolol Hydrochloride and Low Dose of 2DG Decreases the Proliferation of Human A431 Cells
To determine the sensitivity of A431 cells to different concentrations of (±)-Propranolol Hydrochloride and 2DG, we performed the MTT test that establishes cell metabolism by measuring the functionality of mitochondrial dehydrogenases. The effects of (±)-Propranolol Hydrochloride were shown only after 48 h of treatment in all concentrations with a significant effect observed from 200μM. Results revealed that propranolol reduced cell metabolism in a dose-dependent manner (Figure 1A).
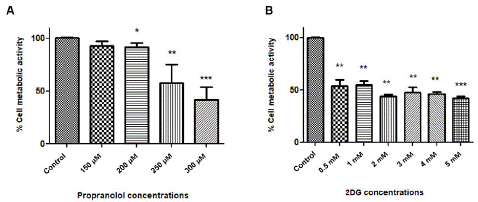
Figure 1: Effect of different concentrations of (±)-Propranolol Hydrochloride and 2DG on squamous carcinoma cell metabolic activity. MTT assay from A431 cell line (A) treated with different concentrations of Prop. (B) A431 cells were treated with different concentrations of 2DG. Cells were treated during 48 h with different concentrations of Prop (150-350μM) and 2DG (0.5-5mM), and an MTT assay was performed. The graphics represent the medium of 3 experiments (*p≤0.05).
Low doses of 2DG were demonstrated to reduce the cells metabolism; however, we did not detect important statistical differences between concentrations from 0.5 to 5mM (figure 1B).
Usually, the aim of combining two or more drugs is to increase the effects, but searching for minimal collateral effects, we tested the minimal effective concentration of both Prop and 2DG. Therefore, all subsequent experiments were performed by using these final concentrations: 200μM propranolol and 0.5mM 2DG.
Because the MTT assay measures the mitochondrial metabolic rate, and it indirectly reflects the viable cell numbers, this assay is commonly performed as a cell proliferation assay. However, to confirm the obtained results of MTT, we performed a cell proliferation assay by counting the alive cells after 48h of treatment. Our results demonstrated that the combination of Prop+2DG determined a significant reduction of A431 cells metabolism (Figure 2A) and proliferation compared to 2DG or propranolol alone (Figure 2B).
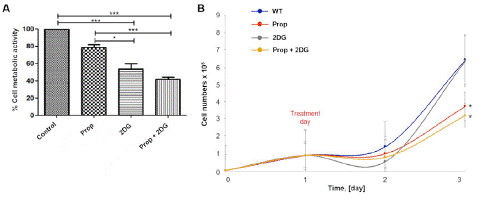
Figure 2: Effect of combined (±)-Propranolol Hydrochloride and 2DG on squamous carcinoma cell A431 metabolic activity and cell proliferation. (A) MTT assay on A431 cell lines treated with combined (±)-Propranolol Hydrochloride and 2DG. A431 cells were treated during 48h with Prop, 2DG or Prop+2DG and MTT assay was performed. (B) A431 cells were seeded and incubated for 24 h when the specific treatments were added. The alive cells were counted once per 24h. The combination of Prop+2DG treatment significantly increased the antiproliferative effect. There was no difference in cell proliferation for the samples treated only with 2DG. The graphics represent the medium of 3 experiments (*p≤0.05).
Ki-67 is an antigen associated with mitosis in mammalian cells, vastly used as a cell proliferation marker. Our results demonstrated that after 24h, a time point in which the differences start to be observed on cell proliferation (Figure 2B), the Ki-67 expression was reduced on treated cells (Figure 3). The decreased expression of Ki-67 in 2DG treatments is consistent with the decrease of cell numbers at 24h (Figure 2B). However, after 48h of 2DG treatment, we observed that tumor cells start to grow again, and the cells come to confluence, which does not allow us to perform the count of positive Ki-67. In tumor cells glycolysis contributes less than 50% for energy production [35], therefore the early effect of 2DG by itself could be circumvented by oxidative phosphorylation’s process.
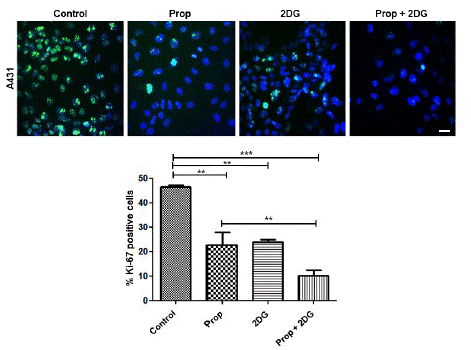
Figure 3: The effect of Prop, 2DG or Prop+2DG on A431 cell proliferation by Ki-67 immunofluorescence assay. A431 cells were treated during 24h with the three treatments. Cells were fixed and labelled with anti-Ki67 (green) and Hoechst 33242 dye (blue). The graphic represents the medium of 3 experiments (*p=0.05). Scale bar = 30μm.
The Combination of Propranolol and 2DG Slightly Increases the Apoptosis while does not Affect the Cell Cycle in A431 Cells
Aiming to ascertain the cytotoxic and/or cytostatic effects of Prop, 2DG or Prop+2DG, we performed apoptosis and cell cycle assays. Our data demonstrated no significant apoptotic role of 200μM, which corroborates with preview studies [36]. The same was observed in the treatment with 0.5mM 2DG, which did not induce apoptotic events. However, the combination of propranolol and 2DG has a slightly superior apoptotic effect than the drugs singularly, but the effect is still very reduced (Figure 4A).

Figure 4: (A) Cell apoptosis assay. A431 cells were treated with Prop, 2DG or Prop+2DG for 48h. The graphic represents the medium of 3 experiments (*p=0.05). The combination of propranolol and 2DG induces higher apoptosis than Prop or 2DG alone. (B) Cell cycle assay. A431 cells were seeded and incubated overnight. In the next day, cells were treated with Prop, 2DG or Prop+2DG for 48 h. The graphic represents the medium of 3 experiments (*p=0.05). The combination of Prop and 2DG slightly increased the number of A431 cells on G2.
Cells singularly treated with (±)-Propranolol Hydrochloride demonstrate a modest increase of G1 phases cells. 2DG alone reduced the percentage of cells in S and G2+M, apparently by arresting cells in G1, which corroborates other studies [37,38]. On the other hand, the combination of Prop+2DG slightly increased the percentage of cells on G2 phase (Figure 4B).
Propranolol Associated with 2DG Reduces HaCaT Cell Metabolism, Growth and Motility, but does not Hinder it
Since we suggest that (±)-Propranolol Hydrochloride could be used in topical formulations for cutaneous SCC therapy pre-chirurgical procedures, to ensure that the use of propranolol did not disturb the wound healing process, which would go against our main goal of reducing healing after tumor removal, we performed a cell migration assay, by using the normal keratinocyte cell line HaCaT.
It was observed that all the propranolol tested concentration and all tested drugs or combination reduce cell metabolism (Figure 5A–5B) and cell proliferation (Figure 5C–5D) of HaCaT.
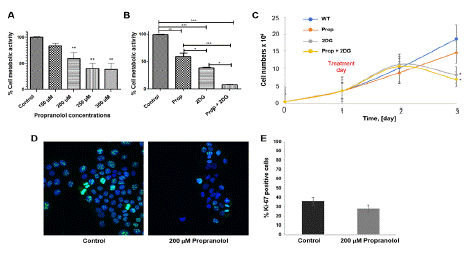
Figure 5: Effect of combined (±)-Propranolol Hydrochloride and 2DG on normal human keratinocytes cell metabolic activity and proliferation. MTT assay from HaCaT cell line treated with: (A) different concentrations of (±)-Propranolol Hydrochloride or (B) Prop, 2DG or Prop+2DG (C) cell growth curve of 72h of culture with 48h of treatment. The alive cells were counted once per 24h. HaCaT cells treated with 2DG and with the combination of 2DG on propranolol significant decrease on proliferation index. (D) The effect of 200μM (±)-Propranolol Hydrochloride on HaCaT cell during 2h by Ki-67 immunofluorescence assay. (E) There was no significant statistical difference between untreated and treated cells. The graphics represent the medium of 3 experiments (*p=0.05). Scale bar = 20μm.
Besides that, to control if propranolol could impair the mobility of normal keratinocytes, we performed a scratch test. When compared to the control (complete culture medium), all treatments reduce the mobility of HaCaT cells. At 40h the complete culture medium induces complete scratch closure, while all treatments induce a partial closure (Figure 6), however at 60h all scratches result close (images not shown). Therefore, the propranolol + 2DG reduces the mobility of HaCaT cells, but do not inhibit it.

Figure 6: Scratch test with normal keratinocyte (HaCaT). Cells were grown up to 100% of confluence when the scratch was made. Cells were treated with Prop, 2DG or Prop+2DG. Cell cultured in complete medium was considered as control. Scale bar = 500μm.
Discussion
The adrenoceptors belong to the G protein-coupled receptor family and are divided into a and β. The β-ARs in turn, are subdivided into three subtypes: β1, β2, and β3 [39]. Their activation triggers a range of transcriptional regulators pathways that modulate the expression of numerous genes including Interleukin-6 (IL-6), Vascular Endothelial Growth ffactor (VEGF), interleukin-8 (IL-8), and matrix metalloproteinases, which promote angiogenesis, cellular invasion, and inflammation [40]. Consequently, an increasing number of studies established, on different tumor types, the β-ARs subtypes, their expression, and their role in cancer processes (Reviewed by Tang J, et al.) [41].
In the present study, we demonstrated that 200μM of the non-selective β-AR (±)-Propranolol Hydrochloride decreases the proliferation of A431 cell line. Even if no substantial statistical differences on cell cycle and apoptosis assay were observed, cells that were treated with 200μM of Propranolol demonstrated an important decrease of Ki-67 expression, which suggests that cell proliferation and growth were affected by the treatment.
However, we verified that the association of Propranolol with the glucose analog 2DG improves the cSCC cells sensitivity to the treatment, by decreasing their metabolism and consequently the proliferation of these cells. It was confirmed by the decrease in the cell growth (about 58%) and in the expression of the Ki-67 (about 78%) after 48h of treatment. We also observed that differently of single treatments, the association of propranolol and 2DG has slightly increased the apoptotic events.
Since it is known that the oral assumption of propranolol can cause some reversible, but inconvenient adverse effects [42], such as lower heart rate, diarrhea, dry eyes, hair loss, nausea, hypoglycemia [43], weakness or tiredness [44,45], we believe that topical application of propranolol could be a promising option for cSCC therapy being a promising strategy to decrease the area of the tumor before surgical removal, avoiding large dimensions wounds caused by the biopsies procedures that tend to be difficult to heal, mainly in the scalp region. The topical application of propranolol is already used for infant hemangioma therapy [26,27], with the advantages of the absence of systemic side effects associated with oral administration. Even the topical use of 2DG can be an effective pharmacological agent if used in an appropriate vehicle and at the proper dosage [46].
However, our results demonstrated that at the concentration of 200μM Propranolol decreased the cell metabolism and growth of the normal keratinocyte cell line HaCaT. Considering these data, we decided to evaluate if the topical use of propranolol and 2DG could impair the closure process. However, the results of the scratch test demonstrated that the treatment increases the time of wound healing closure, but does not hinder it.
Propranolol as a dermatologic therapeutic tool was first described in 2008 [47], and its relatively low adverse risk profile makes it a versatile tool to use both systemically and topically. It was reported that Propranolol blocks the late phase of autophagy then, when in conditions of enhancing autophagy flux, cancer cells demonstrated to be especially sensitive to propranolol [48]. Modulation of autophagy may provide a promising avenue to cancer therapy but considering the complex relationship between autophagy and cancer investigating the effect of propranolol on autophagy in cSCC cell lines would be desirable.
Conclusions
The results obtained represent the first steps to the development of a promising treatment that could be topically applied as an adjuvant pre-surgical therapy against cSCC, aiming to decrease the size of the injury before the surgical procedure, avoiding large wounds or scars, without systemic adverse effects to the patient. These findings should be confirmed on several tumor cutaneous cell line and in vivo experiments should be performed.
Author Statements
Acknowledgements
The authors wish to thank Dr. Vassili Fotis for his private donation which allowed to support this study.
Funding
This work was supported by the private donation of Dr. Vassili Fotis.
References
- Muzic JG, Schmitt AR, Wright AC, Alniemi DT, Zubair AS, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: A population-based study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017; 92: 890-8.
- Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA, et al. Interventions for non-metastatic squamous cell carcinoma of the skin. Cochrane Database Syst Rev. 2010; 14: CD007869.
- Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013; 149: 541-7.
- Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008; 9: 713-20.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008; 58: S129-32.
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991; 88: 10124-8.
- Tsai KY, Tsao H. The genetics of skin cancer. Am J Med Genet C Semin Med Genet. 2004; 131C: 82-92.
- Leblanc KG, Hughes MP, Sheehan DJ. The role of sirolimus in the prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2011; 37: 744-9.
- Paradisi A, Waterboer T, Sampogna F, Tabolli S, Simoni S, et al. Seropositivity for human papillomavirus and incidence of subsequent squamous cell and basal cell carcinomas of the skin in patients with a previous nonmelanoma skin cancer. Br J Dermatol. 2011; 165: 782-91.
- Pierceall WE, Goldberg LH, Ananthaswamy HN. Presence of human papilloma virus type 16 DNA sequences in human nonmelanoma skin cancers. J Invest Dermatol. 1991; 97: 880-4.
- Jaju PD, Ransohoff KJ, Tang JY, Sarin KY. Familial skin cancer syndromes: increased risk of nonmelanotic skin cancers and extracutaneous tumors. J Am Acad Dermatol. 2016; 74: 437-51.
- Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of nonmelanoma skin cancer: systematic review and meta-analysis. Arch Dermatol. 2012; 148: 939-46.
- Jellouli-Elloumi A, Kochbati L, Dhraief S, Ben Romdhane K, Maalej M. Cancers sur cicatrice de brûlure: 62 cas [Cancers arising from burn scars: 62 cases]. Ann Dermatol Venereol. 2003; 130: 413-6.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011; 3: 60-4.
- Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013; 347: f6153.
- Alvi S, Jenzer AC. Scalp reconstruction. StatPearls. 2023.
- Coelho M, Soares-Silva C, Brandão D, Marino F, Cosentino M, et al. β-Adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017; 143: 275-91.
- Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001; 53: 319-56.
- Entschladen F, Drell TL, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004; 5: 254-8.
- Drell TL, Joseph J, Lang K, Niggemann B, Zaenker KS, et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003; 80: 63-70.
- Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001; 61: 2866-9.
- Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control. 2004; 15: 535-41.
- Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010; 1: 628-38.
- Chang PY, Chung CH, Chang WC, Lin CS, Lin HH, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLOS ONE. 2019; 14: e0216828.
- Kashles O, Levitzki A. Characterization of the beta 2-adrenoceptor-dependent adenylate cyclase of A431 epidermoid carcinoma cells. Biochem Pharmacol. 1987; 36: 1531-8.
- Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, et al. Timolol ma-leate 0.5% or 0.1% gelforming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatr Dermatol. 2012; 29: 28-31.
- Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics. 2013; 131: e1739-47.
- Price A, Rai S, Mcleod RWJ, Birchall JC, Elhassan HA. Topical propranolol for infantile haemangiomas: a systematic review. J Eur Acad Dermatol Venereol. 2018; 32: 2083-9.
- Spera G, Fresco R, Fung H, Dyck JRB, Pituskin E, et al. Beta blockers and improved progression free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/ TRIO-012 study. Ann Oncol. 2017; 28: 1836-41.
- Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, et al. Propranolol reduces cancer risk: a populationbased cohort study. Med (Baltim). 2015; 94: e1097.
- Lucido CT, Miskimins WK, Vermeer PD. Propranolol promotes glucose dependence and synergizes with dichloroacetate for anti-cancer activity in HNSCC. Cancers (Basel). 2018; 10: 476.
- Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014; 355: 176-83.
- Shi Z, Li CY, Zhao S, Yu Y, An N, et al. A systems biology analysis of autophagy in cancer therapy. Cancer Lett. 2013; 337: 149-60.
- Bustamante P, Miyamoto D, Goyeneche A, de Alba Graue PG, Jin E, et al. Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med. 2019; 8: 7265-77.
- Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004; 313: 459-65.
- Wang F, Liu H, Wang F, Xu R, Wang P, et al. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol Med Rep. 2018; 17: 5213-21.
- Halicka HD, Ardelt B, Li X, Melamed MM, Darzynkiewicz Z. 2-deoxy-D-glucose enhances sensitivity of human histiocytic lymphoma U937 cells to apoptosis induced by tumor necrosis factor. Cancer Res. 1995; 55: 444-9.
- Zhao J, Ma Y, Zhang Y, Fu B, Wu X, et al. Low-dose 2-deoxyglucose and metformin synergically inhibit proliferation of human polycystic kidney cells by modulating glucose metabolism. Cell Death Discov. 2019; 5: 76.
- Alicia Luthy IA, Bruzzone A, Pérez Piñero CP. Adrenergic action in breast cancer. Curr Cancer Ther Rev. 2012; 8: 90-9.
- Nilsson MB, Le X, Heymach JV. β-Adrenergic Signaling in Lung Cancer: A Potential Role for beta-blockers. J Neuroimmune Pharmacol. 2020; 15: 27-36.
- Tang J, Li Z, Lu L, Cho CH. β-Adrenergic System, a Backstage Manipulator Regulating Tumour Progression and Drug Target in Cancer Therapy. Semin Cancer Biol. 2013; 23: 533-42.
- Khalil RM, El Arini SK, AbouSamra MM, Zaki HS, El-Gazaerly ON, et al. Development of lecithin/chitosan nanoparticles for promoting topical delivery of propranolol hydrochloride: design, optimization and in-vivo evaluation. J Pharm Sci. 2021; 110: 1337-48.
- Holland KE, Frieden IJ, Frommelt PC, Mancini AJ, Wyatt D, et al. Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol. 2010; 146: 775-8.
- Tang LY, Hing JW, Tang JY, Nishikawa H, Shahidullah H, et al. Predicting complications with pretreatment testing in infantile hemangioma treated with oral propranolol. Br J Ophthalmol. 2016; 100: 902-6.
- Kim KH, Choi TH, Choi Y, Park YW, Hong KY, et al. Comparison of efficacy and safety between propranolol and steroid for infantile hemangioma: a randomized clinical trial. JAMA Dermatol. 2017; 153: 529-36.
- University of Maryland center of excellence in regulatory science and innovation (M-CERSI) University of Maryland School of Pharmacy; 2020. Deoxy-D-Glucose. Summary report. 2023.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008; 358: 2649-51.
- Brohée L, Peulen O, Nusgens B, Castronovo V, Thiry M, et al. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci Rep. 2018; 8: 7050.