
Special Article - Melanoma Skin Cancer
Austin J Dermatolog. 2016; 3(1): 1045.
Predictive Biomarkers for Immunotherapy in Melanoma
Rabold K* and Blokx WAM
Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands
*Corresponding author: Rabold K, Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands
Received: December 07, 2015; Accepted: February 19, 2016; Published: February 22, 2016
Abstract
Advanced melanoma is an aggressive tumor that is difficult to treat. Yet new immunotherapeutic strategies are dramatically improving clinical outcomes. Especially Dendritic Cell (DC) vaccination shows promising outcomes at relative low cost when compared to Adoptive Cell Therapy (ACT) and immune checkpoint inhibition. However, not all patients respond to immunotherapy equally and the costly treatments can cause severe toxicities. Therefore, identification of predictive biomarkers to enable selection of patients that are eligible for immunotherapies is of utmost importance in order to improve treatment efficacy and reduce overall cost as well as toxicities. Focus of current investigations lies on the composition of the tumor microenvironment, as an immune-active tumor microenvironment seems to be beneficial for the effect of immunotherapy. Here, we review immunotherapies in melanoma with focus on immune checkpoint inhibition and DC vaccination. Our objective is to give an overview on the recent state of predictive biomarkers for immunotherapy in melanoma.
Keywords: Melanoma; Immunotherapy; Prognosis; Predictive; Marker; Review
Abbreviations
DC-Dendritic Cell; ACT-Adoptive Cell Therapy; APC - Antigen- Presenting Cell; MHC-Major Histocompatibility Complex; TSAs - Tumor-Specific Antigens; TCR- T-Cell Receptor; IFN - Interferon ; CTLA- 4 - Cytotoxic T-Lymphocyte Associated Antigen-4; PD- 1- Programmed Cell Death 1; TME - Tumor Microenvironment; MDSC - Myeloid-Derived Suppressor Cells; ACT - Adoptive Cellular Immunotherapy; CAR - Chimeric Antigen Receptor; FDA - Food and Drug Administration; EMA - European Medicines Agency; OS - Overall Survival; ORR - Overall Response Rate; irAE - Immune- Related Adverse Events; RR - Response Rate; PFS - Progression-Free Survival; TAAs - Tumor-Associated Antigens; pDC - Plasmacytoid Dendritic Cells; DAS – Dasatinib; VEGF - Vascular Endothelial Growth Factor
Introduction
Two emerging hallmarks of cancer, ‘tumor-promoting inflammation’ and ‘avoiding immune destruction’, have been described in a landmark review by Hannah and Weinberg in 2011 [1]. Now it is widely accepted that inflammatory and immune cells can promote cancer outgrowth but can also repress tumor growth [2-4]. This dual role of immunity on tumorigenesis is referred to as cancer immunoediting, a dynamic process consisting of three phases: elimination, equilibrium, and escape [3,5].
Elimination of a developing tumor occurs when molecules and cells of the innate and adaptive immunity identify transformed cells and destroy them on the basis of their expression of Tumor- Specific Antigens (TSAs). This process is also referred to as cancer immunosurveillance [3]. However, the tumor might not always be completely eliminated, can then become dormant and enter into an equilibrium phase where net tumor outgrowth is controlled by the immune system [3]. Finally, tumor cells might be able to progress into the escape phase by immune suppressive effects or when
transformed cells acquire adaptations that allow them to grow in an immunologically unrestricted manner [3,6]. The major cellular mediators in immunosurveillance are CD8+ cytotoxic T-cells and CD4+ T helper cells, next to Dendritic Cells (DCs). In a series of stepwise events, called the cancer-immunity cycle, first neoantigens produced by the cancer cells are recognized by DCs. Next, DCs present the captured antigens on Major Histocompatibility Complex (MHC) molecules to CD8+ T-cells, leading to T-cell activation and production of apoptosis-inducing molecules or cytotoxic granules. Full T-cell activation requires also a co-stimulatory signal of the T-Cell Receptor (TCR) CD28molecule from B7.1 (CD80) or B7.2 (CD86) on the Antigen-Presenting Cell (APC) [7-9]. CD4+ Th1 cells can provide help to the CD8+ T-cells [10]. Both, CD8+ T-cells and CD4+ Th1 cells, restrain carcinogenesis by producing interferon (IFN)-γ and cytotoxins [11].
Nevertheless, tumor cells can develop mechanisms to escape the immune control. One is the inactivation of once activated T-cells [2, 3]. The two best known pathways of T-cell inactivation are the expression of the Cytotoxic T-Lymphocyte Associated Antigen-4 (CTLA-4) receptor on the surface of a T-cell which transducts inhibitory signals from the APC to the T-cell nucleus, and the expression of the Programmed Cell Death 1 (PD-1) receptor on the T-cell surface which may lead to inactivation of the T-cell after binding to its ligands (PD-L1 and PD-L2) on tumor tissue [12]. These are also called immune checkpoints. Secondly, tumors can escape immune control due to immunosuppression by suppressive cells in the Tumor Microenvironment (TME), such as Myeloid-Derived Suppressor Cells (MDSC) or regulatory T-cells (Treg) that produce immunosuppressive molecules [13,14].
With our increasing understanding of cancer immunoediting and increasing knowledge about the tumor microenvironment, new strategies are developed to use the power of immunity for protection against cancer development or cancer progression. The development of cancer immunotherapy has reached an important milestone. Overall survival in patients with advanced metastatic disease has been improved, and - in contrast to targeted cancer therapies - durable monotherapy responses are being reported for different cancer types with several different agents [15-20]. Melanoma is a very aggressive tumor with about 132,000 diagnoses globally each year [21]. Patients are diagnosed with melanoma at the median age of 64 years for men and 57 years for women [22]. Albeit 84% of melanomas are diagnosed in the early stage and are mostly curable, the more advanced stages are still a challenge [22]. Patients with localized melanoma have a 5-year survival of 98.3%, whereas survival rates are radically declining in regional and distant stage disease to 62.4% and 16%, respectively [22]. The median survival duration of patients with metastatic melanoma is poor with only6 to 9 months [23]. Targeted therapies (e.g. BRAF inhibitors) were widely used as first-line treatment of advanced melanoma, but have the drawback that, in most cases, patients will develop resistance [24]. With the emergence of immunotherapy, a shift towards immunotherapy as first-line treatment for advanced melanoma is now observed, since they provide durable tumor control and long-term survival benefits [24].
Here, we review the most successful immunotherapies in melanoma. These include adoptive T-cell therapy, immune checkpoint inhibitors, and cancer vaccines. Our major focus is to describe the current knowledge on predictive biomarkers, which are markers that predict the clinical effect of a specific treatment. They are needed to improve treatment outcomes and to better select patients for these often expensive or laborious treatments to minimize the high costs [25] of immunotherapies.
Immunotherapies in Melanoma
Amongst the most successful strategies of immunotherapy in melanoma are adoptive cell therapy, immune checkpoint inhibitors, and cancer vaccines. The mechanisms, clinical efficacy, and known predictive biomarkers of these approaches are discussed, with emphasis on checkpoint inhibitors and dendritic cell vaccination, which are the most recent and successful developments in immunotherapy in melanoma.
Adoptive Cell Therapy
In the Adoptive Cellular Immunotherapy (ACT), autologous or allogeneic tumor-reactive T-cells are administered to patients, which have the ability to mediate cancer regression. For the cell preparation, lymphocytes with high affinity for tumor antigens are isolated, selected ex vivo, stimulated in vitro, and expanded to achieve sufficient number to eliminate important tumor masses in the patient. In vitro activation allows escape from inhibitory factors that exist in vivo [26]. Immunosuppression by lymphocyte-depleting chemotherapy is performed immediately before T-cell infusion to provide a favorable microenvironment for antitumor immunity [26]. Once the cells are administered, they can proliferate and maintain their antitumor effector functions [26].
Promising results have been shown in melanoma patients, where 49-72% of the patients respond to autologous ACT treatment, depending on the dose of lymph depletion, and 22% show complete tumor regression [27,28]. Since some tumors in this study were rendered inoperable, this approach can be attractive for treatment of tumors that cannot be removed surgically. Nevertheless, the poor outcome of ACT trials in other solid cancers and some limitations of ACT, such as the inability to expand autologous antitumor T-cells, have led to the development of genetic modification of T-cells with either a T-Cell Receptor (TCR) or a Chimeric Antigen Receptor (CAR) [26, 29].
The costs of the preparation vary wildly depending on patient characteristics, the protocol used, and the efficiency of the processing lab and are thus difficult to define. However, the cell generation costs including release testing are estimated around $25,000 to $40,000 per patient [30,31], but additional medical costs such as lymph depletion and treatment of adverse events can inflate the total costs to approximately $90,000 to $100,000per patient [32].
Potential safety risks associated with ACT are on-target off-tumor and off-target activity toxicities, and cytokine-release syndromes, although these are uncommon [33,34]. On-target off-tumor activity occurs when the antigen target is not tumor-specific but also present on nonmalignant cells. In melanoma patients this can result in adverse events such as vitiligo, uveitis or hearing loss when patients are treated with T cells targeting melanocytic differentiation antigens [33,34]. Off-target reactivity can occur as cross-reactivity against peptides in proteins other than the targeted ones, of which only one case is known [34]. Cytokine release syndrome can occur due to high tumor cell lysis leading to high levels of cytokine release and macrophage activation. This can cause high fevers, rigors and hypotension [33] (Table 1).
Adoptive Cell Therapy
Immune Checkpoint Inhibitors
DC vaccination
Ipilimumab
Nivolumab
Pembrolizumab
Efficacy
ORR
49-72% [28, 76]
10.9-11.9% [16, 51]
28-41% [17, 47, 48]
26-38% [15, 50, 51]
37.5% [61]
Median OS
N.K.
9.5-11.4 months [16, 41]
16.8 months [46]
N.K.
13.6-15 months [61-63]
Survival rate
3-year survival 36% (average) – 100% (complete responders) [28]
3-year survival 21% [41],
1-year survival 58% [51]3-year survival 41% [128],
2-year survival 43-48% [46, 128],
1-year survival 58-73% [46, 47, 51]1-year survival 68-74% [51]
3-year survival 68.2% [129]2-year survival 76%,
1-year survival 87% [130]
(all after LND)
6-month PFS rate
N.K.
26.5% [51]
N.K.
34-47% [50, 51]
N.K.
Median PFS
7.6 months [76]
2.8-2.9 months [51, 54]
6.9 months [54]
4 - >7 months [15, 51]
5-34.4 months [62, 64]
Average Costs
~ $100,000/patient [30-32]
$92,500[31]- $158,252/ patient [42, 43]
$103,220/patient [42]
$150,000/year [131]
$9,000-20,000/ patient [71, 72]
Toxicity
On-target off-tumor reactivity
(e.g. skin rash, vitiligo, uveitis, hearing impairment, seizures) [34, 132]Off-target reactivity
(diverse unpredictable autoreactivities) [34]Cytokine release syndrome (fever, rigor, hypotension, hypoxia) [132]
Incidence (grade 3-5):
5-27.3% [16, 38, 51, 54]Fatigue (34-41%),Diarrhea (27-37%),
Pruritus (21-31%)
Rash (25-29%),
Colitis (5-8%) [16]
Incidence:
5-16.3% [47, 48, 54]Fatigue (20-24%),
Pruritus (16-17%),
Nausea (16.5%) [47, 48]
Incidence (grade 3-5):
10.1-13% [51]Fatigue (20.9%),
Diarrhea (16.9%),
Rash (14.7%)
Pruritus (14.4%) [51]
Mild (˂ grade 3) or none [62, 63, 66, 67, 70] Local skin reaction at injection site (31-100%),
Fever (4-20%),
Vitiligo (3-30%) [62, 63, 66-70]
, Dendritic Cell; ORR, Overall Response Rate; OS, Overall Survival; PFS, Progression-Free Survival; LND, Lymph Node Dissection; N.K., Not Known
Table 1: Efficacy, Costs and Toxicity of Immunotherapies in Melanoma.
Immune checkpoint inhibitors
Tumors can escape immune control by regulating the function of T-cells via inhibitory pathways. These immune checkpoints normally function to control excessive immune activation. Blockade of the immune checkpoints with monoclonal antibodies against CTLA-4 or PD-(L)1 (Figure 1) seems to be beneficial in tumors, especially in melanoma.
CTLA-4 Antibodies: CTLA-4 is a co-inhibitory receptor that is expressed on the surface of CD4+ and CD8+ T-cells after T-cell activation. By binding B7.1 (CD80) and B7.2 (CD86) with a higher avidity than the TCR co-stimulatory molecule CD28, CTLA-4 can inhibit CD28-dependent T-cell immune response [35]. Blockade of CTLA-4 is thought to release the inhibition of T-cell activation, thus enhancing T-cell activation, proliferation and function, including IFN-γ production [35] (Figure 1). In this way, CTLA-4 antibodies potentiate ongoing immune responses, including cancer immunosurveillance [36].
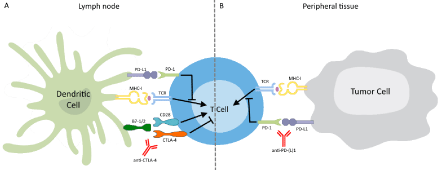
Figure 1: Schematic of Immune Checkpoint Inhibition.
The interaction of a T-Cell Receptor (TCR) with a presented antigen starts
the process of immune activation, for which also CD28 co-stimulation is
required. (A) CTLA-4 mediated inhibition can dampen the immune response.
Inhibition of CTLA-4 by a monoclonal antibody counters this inhibition and
allows unregulated T-cell activation. (B) PD-1, upon binding to its ligand PDL1
on the tumor cell, activates an inhibitory pathway in the T-cell. Blocking
the interaction between receptor and ligand with a monoclonal antibody down
regulates the inhibitory stimulus.
Ipilimumab: Ipilimumab, a fully humanized CTLA-4 antibody, was developed and entered clinical trials in 2000 [37]. It showed promising response in phase I and II trials with metastatic melanoma patients [38,39] and was approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2011, after a phase III randomized clinical trial showed positive results.
In this study, patients with unresectable stage III or IV melanoma were randomized 3:1:1 into three arms, ipilimumab plus glycoprotein 100 (gp100), ipilimumab alone or gp100 alone [16]. The results show that ipilimumab has clinical benefits, as the median Overall Survival (OS) for both Ipilimumab-arms was significantly higher than for gp100 alone (10 versus 6.4 months). Furthermore, Overall Response Rate (ORR) was highest in ipilimumab alone [16,35]. In a second large phase III randomized study, the efficacy of ipilimumab was compared to chemotherapy (dacarbazine). Previously untreated metastatic melanoma patients were randomly assigned 1:1 into two arms of the study, ipilimumab plus dacarbazine or dacarbazine alone [40]. The combination showed significantly longer OS than the dacarbazine monotherapy (11.2 versus 9.1 months), with higher 1-3 year survival rates in the ipilimumab-dacarbazine group [40]. Both phase III clinical trials demonstrated improved OS in patients with advanced melanoma treated with ipilimumab. Recently, a pooled analysis of OS data from multiple studies provided a more precise estimate of long-term survival for ipilimumab-treated patients with advanced melanoma. The analysis performed on 1861 patients showed that survival reaches a plateau at a survival rate of 21% beginning around year 3, independent of prior therapy or ipilimumab dose [41]. Immune-Related Adverse Events (irAE) occurred most often during the third or fourth treatment-cycle, with the most common being fatigue, diarrhea, pruritus and skin rash [16]. Although most irAEs are reversible with appropriate treatment, they can be severe, longlasting and in some cases even lethal (2.1%) [16]. Costs for ipilimumab treatment vary from $92,500 - $158,252 per patient [31,42,43].
PD-1 and PD-L1 Antibodies: In contrast to CTLA-4, PD-1 does not interfere with co-stimulation, but with signaling downstream of the TCR. It is expressed on CD8+ and CD4+ T-cells as well as B-cells and interacts with PD-1 ligand 1 or 2 (PD-L1 (B7-H1) and PD-L2 (B7-DC)) to regulate the threshold of antigen responses of T-cells and B-cells in peripheral tissues, i.e. at the tumor site (Figure 1). This interaction inhibits the proliferation and effector function of T-cells, leading to decreased cytokine production and antibody formation [44]. Whilst highly regulated under physiological conditions, the expression of these ligands can be upregulated in tumor cells, thereby diminishing the strength of the T-cell response [17]. By inhibiting PD-1 receptors or its ligands, effector phase T-cell responses are reinforced [36]. Several antibodies targeting PD-1 (nivolumab, pembrolizumab (MK-3475)), both approved by the FDA in 2014, or PD-L1 (MPDL3280A, MEDI4736, etc.) are in different stages of development.
Nivolumab: Nivolumab is a fully human IgG4-isotype monoclonal antibody against PD-1. It has been approved by the FDA in December 2014 [12] and by the EMA in June 2015 for the treatment of unresectable or metastatic melanoma unresponsive to other drugs [45]. The approval was based on the results of a clinical trial published in 2014 [46]. 31% of the 107 patients with advanced melanoma had an objective response, and the median response duration was 2 years. Median OS was 16.8 months, and 1- and 2-year survival rates were 62% and 43%, respectively [46]. The first published phase III clinical trial was a comparison of nivolumab to chemotherapy (dacarbazine) in previously untreated patients without BRAF-positive melanoma [47]. The 1-year OS was 72.9% with nivolumab versus 42.1% with chemotherapy and the ORR was noticeably higher with nivolumab (40%) than in the dacarbazine group (13.9%) [47]. Similar ORRs were described in another phase III study comparing nivolumab to chemotherapy (dacarbazine) in patients that progressed after ipilimumab or ipilimumab and BRAF inhibitor treatment [48]. Both studies showed a better safety profile in the nivolumab group with 11.7% or 5% adverse events versus 17.6% or 9% in the chemotherapy group, respectively. Most common treatment-related adverse events were fatigue, pruritus, and nausea [47,48]. Costs for treatment with nivolumab are around $100,000 per patient [42].
Pembrolizumab/Lambrolizumab (MK-3475): Pembrolizumab (formerly lambrolizumab or MK-3475) is a highly selective humanized IgG4-isotype monoclonal antibody against PD-1. Of the PD-1 directed antibodies, pembrolizumab has the highest affinity for PD-149. It was the first anti-PD-1 drug that got approval from the FDA for treatment of melanoma patients with relapsed or refractory disease in September 2014. A cohort study with advanced melanoma patients reported a high dose-dependent Response Rate (RR) (median ORR was 38%, RR with high dose was 52%) and an overall median Progression-Free Survival (PFS) that was longer than 7 months [15]. A recent phase II study with ipilimumab-refractory melanoma patients showed that pembrolizumab improves PFS compared to chemotherapy. 6-months PFS rates were more than 30% in pembrolizumab-treated patients compared to 16% for chemotherapy [50].
Comparison of pembrolizumab with ipilimumab in a phase III study with advanced melanoma patients revealed that pembrolizumab increased 6-month PFS (more than 45% versus 26.5% for ipilimumab) and 1-year survival rates (Table 1) [51]. This study suggests that pembrolizumab may be more efficacious than ipilimumab, while the costs are comparable (Table 1). Overall, PD-1 inhibition (nivolumab or pembrolizumab) shows a higher efficacy with higher response rates and durable responses as well as less adverse events than CTLA- 4 inhibition (ipilimumab) (Table 1).
Combination CTLA-4 and PD-1 Inhibition: Several studies have been performed testing the combination of nivolumab with ipilimumab to examine whether a synergistic effect would occur. Two phase II randomized trials showed that response rates were higher in the combination groups (40% and 61%) than with nivolumab (20%) or ipilimumab (11%) monotherapy, respectively [52,53]. The first phase III trial comparing the combination of nivolumab and ipilimumab versus each as monotherapy in treatment-naïve patients was published recently. Nivolumab - either in combination (11.5 months) or alone (6.9 months) - significantly improved median PFS, compared to ipilimumab alone (2.9 months) [54].
In conclusion, the efficacy of nivolumab and ipilimumab combination therapy is superior over single immune checkpoint therapy. Although this was accompanied by an increase in toxicity (grade 3-4 AE in 55% of patients) [53,54], the combination therapy got FDA approval in October 2015 for patients with BRAF V600 wild-type advanced melanoma [55]. This combination therapy would approximately cost more than $200,000 per patient. Interestingly, in PD-L1-negative patients the combination of PD-1 and CTLA- 4 blockade (nivolumab and ipilimumab) was more effective than both monotherapies [54]. PD-L1 expression could thus be useful in determining which patients should receive combination therapy.
Cancer Vaccines
The purpose of cancer vaccines is to stimulate the cancerimmunity- cycle, triggering a long-lasting adaptive immune response, mostly after surgical resection of the melanoma. Vaccines can be based upon antigens ranging from peptides to whole irradiated cancer cells [56,57]. For example, cancer vaccines have been developed using peptides or proteins, which are Tumor-Associated Antigens (TAAs) or are associated with tumor progression, such as gp100 for melanoma. Also whole, inactivated autologous or allogeneic cancer cells can be used for cancer vaccines [56].
The most common approach in melanoma research is Dendritic Cell (DC) vaccination. DC vaccines are created by stimulation of autologous DCs via pulsing with a proper Ag source in vitro [56,57]. DCs are central players in regulation of T cell-stimulation, bridging the innate and adaptive immune responses. In vivo, endogenous DCs process antigens in their immature form are signaled to mature, and migrate to lymph nodes. When maturated they lose their motility and antigen processing capabilities, co-localize with T-cells and activate them [58].
To create DC vaccines, DCs are mostly derived from peripheral blood mononuclear cells, but first clinical studies show that naturally occurring Plasmacytoid Dendritic Cells (pDC) can be handled as well [59]. Different means for pulsing of DCs have been used, including autologous or allogeneic tumor lysate pulsed DCs, peptide cocktailpulsed DCs, mRNA transduced DCs, DC/tumor cell fusion products, and virus-transduced DCs [60]. An autologous tumor lysate pulsed DC vaccine was evaluated in a phase II clinical trial of 24 subjects with stage IV melanoma. The ORR to vaccination was 37.5% and median OS was 15 months [61]. In a study with peptide-pulsed DCs (either MHC-I–restricted gp100 or both MHC-I-restricted gp100 and MHCII- restricted tyrosinase) in 29 patients with advanced melanoma, median OS likewise rose to 15 months and clinical responses improved upon MHC-I/II-loaded DC vaccination [62]. Peptide cocktail-pulsed DC vaccination, performed in a phase II clinical trial in 24 patients with metastatic melanoma, significantly prolonged OS in DC-given melanoma patients (13.6 months in vaccinated vs. 7.3 months in non-vaccinated) [63]. Vaccination with mRNA electroporated DCs in stage III or VI melanoma patients was shown to induce IFN-γ producing CD8+ and CD4+ T-cell responses, which are associated with improved Progression-Free Survival (PFS) and OS [64,65]. In contrast to ACT and immune checkpoint inhibition, adverse events in DC vaccination are absent or only mild, such as local skin reactions at the injection site, fever, or vitiligo [62,63,66-70], and the cost of treatment are considerably lower, namely $9,000 - $20,000 per patient [71,72].
Predictive Biomarkers
Since the response to immunotherapy can vary, immunotherapy can cause serious autoimmune toxicity, and the costs of most treatments are high and are even increasing in case of severe toxicity requiring medical attention, the need for predictive biomarkers is urgent. As accumulating evidence shows that the local antitumor immune response can influence the efficacy of immunotherapies, several studies aimed at identifying potential predictors of response to anti-tumor immunotherapies. Especially the number and type, but also the distribution and location of Tumor-Infiltrating Lymphocytes (TIL) seem to have prognostic value and may help predict response to therapy. This has led to the development of an ‘immunoscore’, a quantification of the density and location of immune-cells within the tumor [73], primarily cytotoxic (CD8+) and memory (CD45RO) T-cells in the center and the invasive margin of tumors [74]. Furthermore, the location of immune cells in adjacent Tertiary Lymphoid Structures (TLS) can also have prognostic value [75] and potentially predictive value.
Adoptive Cell Therapy
Since not all patients respond to ACT in the same way, predictive biomarkers are needed to prevent high costs and life-threatening side effects associated with the therapy [37]. However, little is known about predictive biomarkers in ACT. No associations were found between patient gender, age, tumor stage, and serum Lactate Dehydrogenase (LDH) at time of TIL transfer and clinical response [76]. Also CD62L, which governs the circulation of CD8+ T-cells between lymph nodes and peripheral tissues, was shown not to be predictive for response in a mouse model, although previous studies correlated CD62L expression with the success rate of ACT [77]. Kronik et al. show in their mathematical model, simulating 4 independent clinical trials, that tumor growth rate and tumor load are critical for the success of T-cell therapy in melanoma and thus for predicting the outcome. On basis of this analysis, a large tumor burden might be handled as exclusion criteria if a large CTL dose is unavailable [78].
A higher number of Tumor-Infiltrating Lymphocytes (TIL), and a higher number and percentage of CD8+T-cells in the infusion product is shown to be significantly associated with response in metastatic melanoma. Responding patients showed a higher number of differentiated effecter T-cells and CD8+ T-cells expressing the inhibitory receptor B- and T-Lymphocyte Attenuator (BTLA) than non-responders [76,79]. BTLA expression is thought to mark a specific stage of differentiation and seems to contribute to a longer survival of CD8+T-cells, which might explain the correlation of BTLA expression on CD8+T-cells and clinical response in ACT [79]. Recently, multispectral imaging of the tumor microenvironment in 17 melanoma patients showed that the presence of CD8+T-cells alone was insufficient to predict the successful generation of a TIL culture, a major hurdle in ACT, whereas the ratio of CD8+ T-cells to CD3+ FoxP3+ regulatory T-cells was highly predictive [80].
Immune checkpoint inhibitors
Several studies have been published that investigated potential biomarkers associated with responsiveness to checkpoint inhibition, mainly with regard to gene expression profiles or immune cell infiltration in the local tumor microenvironment, but also systemic factors have been examined. Furthermore, the potential effect of NRAS or BRAF mutation status on the response to checkpoint inhibition was studied recently. The results show that patients with NRAS mutation respond better to immunotherapy, especially to first-line immunotherapy, and show a higher clinical benefit as well as a longer PFS [81]. However, Mangana et al. showed that while the median OS of NRAS mutated patients is prolonged, the difference is not significant [82]. The NRAS mutation status might thus give an indication for a patient’s response to checkpoint inhibition, but can probably not be considered as a predictor of response. Some studies also associate clinical autoimmune responses, especially vitiligo, colitis or pruritus, occurring in patients undergoing immunotherapy for melanoma, with response to checkpoint inhibition [83-86].
CTLA-4 Antibodies: With regard to CTLA-4 blockade, most publications studied prediction of response to the approved ipilimumab, whereas very few studies focused on tremelimumab. Patient age, gender, tumor histology, primary tumor site, and previous therapies show no significant correlation with clinical response in treatment with ipilimumab [87]. Correlations between those metrics and clinical response are as of yet not reported in tremelimumab treatment. Furthermore, somatic mutations and candidate neoantigens were analyzed from melanoma patients treated with ipilimumab or tremelimumab. Whilst the correlation between mutational load and survival was not as strong, shared tetrapeptide neoepitope signature (101 shared tetra peptides) was associated with clinical benefit [88]. Moreover, immune-related adverse events, including colitis, hypophysitis, arthritis and thyroiditis, were associated with significant clinical benefit in patients receiving anti- CTLA-4 therapy [89].
Ipilimumab: There are currently no confirmed biomarkers that are predictive for response to ipilimumab. Initially it was thought that the HLA status could correlate with ipilimumab benefit, but an analysis of four melanoma trials revealed that response to ipilimumab was HLA independent [90].
Several studies analyzed easy accessible blood or serum parameters. High peripheral blood TCR diversity was associated with clinical benefit in a small patient cohort [91]. Moreover, melanoma patients expressing NY-ESO-1 in the serum with associated CD8+ T-cells were found to have more frequent clinical benefit and significant survival advantage [92]. Furthermore, lower baseline levels of circulating Myeloid-Derived Suppressor Cells (MDSCs) were reported to significantly correlate with tumor response [93,94], and OS [95]. High baseline serum concentrations of soluble CD25 (sCD25) predicted resistance to ipilimumab in melanoma patients [96]. Interestingly, low baseline levels of LDH, involved in tumor initiation and metabolism, were also frequently associated with improved OS [97-100]. Similarly, high baseline serum levels of Vascular Endothelial Growth Factor (VEGF) were reported to have a deleterious impact on the chance to experience a clinical response after ipilimumab [101]. The predictive values of these parameters need to be confirmed in larger cohort studies
Currently, Tumor-Infiltrating Lymphocytes (TILs) are studied extensively in melanoma, since they are known to have a prognostic value and might predict efficacy of immunotherapy [102]. Tarhini et al. describes a tendency for association of low baseline levels of CD20+ B-cells in tumor biopsies with worse clinical response to ipilimumab [103]. Furthermore, melanoma-specific T-cells have been shown to have a prognostic impact in metastatic melanoma patients [104,105] and increase upon ipilimumab treatment [103]. In a phase II biomarker study, immunohistochemistry and histology analysis on pretreatment tumor biopsies from patients with unresectable melanoma revealed that high baseline expression of the T regulatory-specific transcription factor fork head box P3 (FoxP3) and the immuno modulatory enzyme Indoleamine 2,3-Dioxygenase (IDO) on tumor-infiltrating mononuclear leukocytes is significantly positively associated with ipilimumab clinical activity [106]. No such association was observed with total infiltrate, expression of CD4, CD45RO, CD8, granzyme B, or perforin [106]. In the same trial, gene expression profiling revealed that high baseline expression levels of immune-related genes, including cytotoxic T-cell surface markers, Th1 cytokines and chemokines, immune-receptors, cytotoxic factors, and T-cell receptors, were associated with response to ipilimumab [107]. Whole-exome sequencing of pretreatment melanoma tumor biopsies showed a significant association between overall mutation, neoantigen load, and cytolytic markers with clinical benefit. However, mutational load alone appears not to be sufficient to predict benefit [88] and no recurrent neoantigen peptide sequences were found to be predictive for response [87].
In addition to lymphocytes, neutrophils and eosinophils are also associated with response to ipilimumab. Baseline eosinophil count in blood was reported to be predictive for outcome upon ipilimumab treatment. A baseline absolute eosinophil count ≥100/μL or baseline relative eosinophil counts of 1.75% were both significantly associated with improved OS [108]. Recently, a low baseline neutrophil-tolymphocyte ration was shown to significantly improve PFS and OS [97,109,110]. Furthermore, several studies and case reports show an association between immune-related adverse events, such as ileitis, colitis and vitiligo, and response to ipilimumab treatment [111-115].
PD-1 and PD-L1 Antibodies: In contrast to ipilimumab, little data is available concerning potential predictive biomarkers during PD-1 or PD-L1 blockade. Evaluation of PD-L1 expression on tumor cells as a predictive marker revealed to be inconclusive. Although some reports mention that PD-L1 expression correlates with response to PD-1 blockade, there is considerable overlap between PD-L1-positive and -negative tumors, regarding clinical response [15,17,47,48,116,117). Currently, the focus lies on immunoprofiling and gene expression sequencing of tumor tissue. Tumors with a high mutational load have a better response to PD-1 blockade [118,119], similar as to what Snyder et al. reported for CTLA-4 immunotherapy. Recently, Campesato et al. integrated the data published by Snyder et al. 2014 and Rizvi et al. 2015 to determine if gene panels could be applied to estimate clinical benefit to checkpoint inhibition [120]. A high mutational load was significantly associated with PFS in patients treated with PD-1 immunotherapy and might thus be useful as a predictor for clinical benefit.
Next to mutational load, also the density of CD8+T-cells at the invasive margin of the tumor may predict response to PD-1 blockade, since pre-treatment samples from patients responding to pembrolizumab showed higher numbers of CD8+ cells [117]. As this predictive model was shown to accurately predict 9 out of 9 patients in the true response group and 4 out of 5 patients in the true progression group [117], CD8+ cell density at the invasive margin seems a promising predictive marker. Interestingly, a case of an advanced melanoma patient is known that displayed vitiligo prior to immunotherapy and showed a significant rapid response to nivolumab treatment [121]. Also in patients receiving pembrolizumab, cutaneous adverse events, such as vitiligo and pruritus were associated with a better objective response to treatment and longer PFS, respectively [83,84].
DC Vaccination
The identification of potential predictive biomarkers in DC vaccination is only at its beginning. In 2011 it was shown that several features can improve survival after DC vaccination in melanoma patients, amongst which younger age (<50 years), male gender, prior radiation therapy, IFN-γ-induced apoptosis, baseline LDH in the normal range, and an Eastern Cooperative Oncology Group performance status of 0 (fully active). Expression of specific antigens (HMB-45, Mel-5, tyrosinase, S100, Mage-1) on the cultured melanoma cells of patients was not associated with difference in survival [122]. Special interest now lies on the tumor microenvironment and tumor-infiltrating lymphocytes, especially on T-cells. A study in melanoma patients receiving autologous tumor cell vaccination showed that a significantly higher number of activated T-cells at baseline were associated with non-progression. This study by Tijn et al. further showed a correlation between CD4, CD8, and granzyme B with OS, too [14]. These findings suggest that sufficient numbers of (activated) T-cells in melanoma tissue at baseline may be beneficial for immunotherapy response. However, the predictive value for response to DC vaccination still needs to be investigated. In contrast, no correlation with OS and suppressive cells, such as regulatory T-cells or Myeloid-Derived Suppressor Cells (MDSC), T-cell inhibitory factors or loss of HLA class-1/ melanocytic differentiation antigens was detected by Tijn et al.[14]. The premise that CD8+ T-cells are beneficial for clinical outcome after DC vaccination is endorsed by findings in a melanoma mouse model, in which the combinatorial therapy of Dasatinib (DAS) and DC vaccination led to elevated CD8+ T effector cell levels, inhibited tumor growth, and extended OS compared to monotherapy. Furthermore, pro-inflammatory cytokine and chemokine expression levels, including IFN-γ, were highest in the combination therapy. DAS, a multi-kinase inhibitor, is suggested to enhance CD8+ T-cell infiltration into the tumor microenvironment and thereby create beneficial circumstances for the functioning of DC vaccination [123]. Next to immunohistochemical analysis, gene expression profiling of pre-treatment biopsies of melanoma metastases can correlate with outcome after immunotherapy, e.g. due to high expression levels of immunosuppressive genes or specific chemokines [124,125]. A DC vaccine trial revealed that pre-treatment melanoma metastases biopsies from favorable outcome patients expressed higher levels of T-cell-specific genes, chemokines, as well as other immune genes [126]. It is suggested that, in a subset of melanomas, the recruitment of activated T-cells into the tumor microenvironment is supported through the regulation of chemokines, which may improve the clinical response to cancer vaccines [124].
In metastatic renal cell carcinoma patients, responders to DC vaccination showed significantly higher cytotoxic effects of peripheral blood lymphocytes compared to cells from non-responders, even before vaccination [127]. Thus, cytotoxic activity is a potential predictive marker, which should be evaluated in prospective studies.
Discussion
Immunotherapy has been shown to be an effective treatment for some patients with melanoma. The various strategies are superior to targeted therapies by providing durable tumor control and longterm survival, making them emerging first-line treatment options to clinicians. Especially dendritic cell vaccination is a very promising approach, considering its relative low cost, low toxicities, and longlasting response.
Although immunotherapies have a high clinical efficacy, not all patients benefit equally and questions remain as to which patients are eligible for a particular immunotherapy. The need for predictive biomarkers, which can help to improve treatment efficacy and costefficiency, is evidently high. Our review reveals that the classical TNM staging characteristics of the primary melanoma are not predictive for response to immunotherapy in various studies, whereas a low tumor growth rate and tumor load with a normal LDH level at baseline were shown to have predictive value in some studies. Also specific antigens, such as melanocytic differentiation antigens, are suggested not to be predictive. Current studies point to a predictive value of tumor immune infiltration, chemokine signature, tumor burden, and tumor mutational load, either alone or in combination. A high mutational load is thought to increase the chance for the immune system to recognize these tumors with a higher neoantigen load, but was in most studies only predictive in combination with other markers. Specific chemokines are suggested to enhance immunosurveillance by recruiting activated T-cells to the tumor microenvironment. Of special interest are the type and number of specific lymphocyte subsets, either in the blood or in the tumor microenvironment. Higher densities of TILs, especially of activated cytotoxic T-cells are indicated to be predictive biomarkers to immunotherapy. In contrast, immunosuppressive subsets are associated with a poor prognosis. An immune-active tumor microenvironment thus seems to be beneficial for the effect of immunotherapy. However, most of these studies were retrospective analyses, operating with incomplete data. Since prospective validation studies in large cohorts are lacking, none of the identified candidate biomarkers are broadly used in daily clinical care and influencing treatment decisions. Next to large sample sizes, further investigation of biomarkers will moreover require clear definitions and agreements (e.g. regarding cut-off values for PDL1 expression), since there are currently considerable differences between studies.
In future studies, it might be worthwhile to analyze T-cell infiltration in depth, taking not only the number or density of cells but also the T-cell subsets, distribution and location (intratumoral, peritumoral, at the center of the tumor, or at the invasive margin) into consideration. Moreover, the predictive value of candidate biomarkers might be enhanced by combination of markers. As shown for ACT, the ratio of cytotoxic T-cells to regulatory T-cells can hold a higher predictive value than the single values [80], which might be true for other immunotherapeutic strategies as well. Combination of various markers, e.g. by multispectral imaging, can create a predictive fingerprint that can hold more information than single parameters.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144: 646-674.
- Giraldo NA, Becht E, Vano Y, Sautès-Fridman C, Fridman WH. The immune response in cancer: from immunology to pathology to immunotherapy. Virchows Arch. 2015; 467: 127-135.
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011; 29: 235-271.
- Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015; 26: 1813-1823.
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011; 331: 1565-1570.
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015; 35: 185-198.
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012; 12: 269-281.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39: 1-10.
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006; 90: 297-339.
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4 (+) T cells in the antitumor immune response. J Exp Med. 1998; 188: 2357-2368.
- Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011; 7: 651-658.
- Niezgoda A, Niezgoda P, Czajkowski R. Novel Approaches to Treatment of Advanced Melanoma: A Review on Targeted Therapy and Immunotherapy. Biomed Res Int. 2015; 2015: 851387.
- Zou W1. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005; 5: 263-274.
- Tjin EP, Krebbers G, Meijlink KJ, van de Kasteele W, Rosenberg EH, Sanders J, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res. 2014; 2: 538-546.
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013; 369: 134-144.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363: 711-723.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366: 2443-2454.
- Mathios D, Park CK, Marcus WD, Alter S, Rhode PR, Jeng EK, et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer. 2016; 138: 187-194.
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J clin oncol : official journal of the American Society of Clinical Oncology. 2015; 33: 2780-2788.
- John T. Role of immunotherapy in lung cancer: Preliminary results of new vaccines and immune checkpoint inhibitors. Asia Pac J Clin Oncol. 2015; 11: 2-8.
- WHO. Ultraviolet Radiation and the INTERSUM Programme. WHO. 2009.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014; 64: 252-271.
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007; 109: 455-464.
- Dummer R, Schadendorf D, Ascierto PA, Larkin J, Lebbé C, Hauschild A. Integrating first-line treatment options into clinical practice: what's new in advanced melanoma? Melanoma Res. 2015; 25: 461-469.
- Geynisman DM, Chien CR, Smieliauskas F, Shen C, Shih YC. Economic evaluation of therapeutic cancer vaccines and immunotherapy: a systematic review. Hum Vaccin Immunother. 2014; 10: 3415-3424.
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015; 348: 62-68.
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J clin oncol: official journal of the American Society of Clinical Oncology. 2005; 23: 2346-2357.
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011; 17: 4550-4557.
- Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene-modified T cells: From the bench to the clinic. Mol Immunol. 2015; 67: 46-57.
- Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011; 17: 1664-1673.
- Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, et al. TCR-Engineered T Cells Meet New Challenges to Treat Solid Tumors: Choice of Antigen, T Cell Fitness, and Sensitization of Tumor Milieu. Front Immunol. 2013; 4: 363.
- Retel VP, Steuten LMG, Mewes JC, van Harten WH. Early Cost-Effectiveness Modeling for Tumor Infiltrating Lymphocytes (TIL) - Treatment versus Ipilimumab in Metastatic Melanoma Patients. Value in Health. 2014; 17.
- Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015; 8: 337-350.
- Tey SK. Adoptive T-cell therapy: adverse events and safety switches. Clin Transl Immunology. 2014; 3: 17.
- Roddie C, Peggs KS. Emerging options for the treatment of melanoma – focus on ipilimumab. ImmunoTargets & Therapy. 2014; 3: 67-78.
- Palathinkal DM, Sharma TR, Koon HB, Bordeaux JS. Current systemic therapies for melanoma. Dermatol Surg. 2014; 40: 948-963.
- Ascierto ML, Melero I, Ascierto PA. Melanoma: From Incurable Beast to a Curable Bet. The Success of Immunotherapy. Front Oncol. 2015; 5: 152.
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010; 11: 155-164.
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of Ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010; 21: 1712-1717.
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364: 2517-2526.
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long-Term Survival Data from Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J clin oncol: official journal of the American Society of Clinical Oncology. 2015; 33: 1889-1894.
- Andrews A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. Am Health Drug Benefits. 2015; 8: 9.
- Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T. 2012; 37: 503-530.
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015; 348: 56-61.
- Squibb B-M. Press release 2015.
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014; 32: 1020-1030.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372: 320-330.
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015; 16: 375-384.
- Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin Ther. 2015; 37: 764-782.
- Dummer R, Daud A, Puzanov I, Hamid O, Schadendorf D, Robert C, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with Ipilimumab-refractory melanoma. J Transl Med. 2015; 13.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015; 372: 2521-2532.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med. 2013; 369: 122-133.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab versus Ipilimumab in untreated melanoma. N Engl J Med. 2015; 372: 2006-2017.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015; 373: 23-34.
- Administration USFaD. Nivolumab in combination with Ipilimumab 2015.
- Suckow MA. Cancer vaccines: harnessing the potential of anti-tumor immunity. Vet J. 2013; 198: 28-33.
- Aris M, Barrio MM, Mordoh J. Lessons from cancer immunoediting in cutaneous melanoma. Clin Dev Immunol. 2012; 2012: 192719.
- Yang JC. Melanoma vaccines. Cancer J. 2011; 17: 277-282.
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013; 73: 1063-1075.
- Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, et al. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012; 1: 1111-1134.
- Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, et al. Unexpected high response rate to traditional therapy after dendritic cell-based vaccine in advanced melanoma: update of clinical outcome and subgroup analysis. Clin Dev Immunol. 2010; 2010: 504979.
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, et al. Targeting CD4 (+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013; 73: 19-29.
- Oshita C, Takikawa M, Kume A, Miyata H, Ashizawa T, Iizuka A, et al. Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial. Oncol Rep. 2012; 28: 1131-1138.
- Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012; 18: 5460-5470.
- Van Nuffel AM, Benteyn D, Wilgenhof S, Corthals J, Heirman C, Neyns B, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemo refractory stage IV-M1c melanoma patient. Cancer immunology, immunotherapy: CII. 2012; 61: 1033-1043.
- Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001; 61: 6451-6458.
- Wilgenhof S, Van Nuffel AM, Benteyn D, Corthals J, Aerts C, Heirman C, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013; 24: 2686-2693.
- Di Nicola M, Carlo-Stella C, Mortarini R, Baldassari P, Guidetti A, Gallino GF, et al. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34(+)-derived dendritic cell vaccination: a phase I trial in metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004; 10: 5381-5390.
- Mackensen A, Herbst B, Chen JL, Kohler G, Noppen C, Herr W, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34 (+) hematopoietic progenitor cells. Int J Cancer. 2000; 86: 385-92.
- Wilgenhof S, Van Nuffel AM, Corthals J, Heirman C, Tuyaerts S, Benteyn D, De Coninck A. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011; 34: 448-456.
- van Hengel W. Vaccinatie tegen kanker. Reformatorisch Dagblad. 2004 03/06/2004.
- Krebsabwehr eVGfB. Tumorimpfung Biologische Krebsabwehr: Gesellschaft für Biologische Krebsabwehr. 2012.
- Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012; 10: 1.
- Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, et al. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013; 11: 54.
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012; 12: 298-306.
- Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012; 18: 6758-6770.
- Díaz-Montero CM, Zidan AA, Pallin MF, Anagnostopoulos V, Salem ML, Wieder E, et al. Understanding the biology of ex vivo-expanded CD8 T cells for adoptive cell therapy: role of CD62L. Immunol Res. 2013; 57: 23-33.
- Kronik N, Kogan Y, Schlegel PG, Wölfl M. Improving T-cell immunotherapy for melanoma through a mathematically motivated strategy: efficacy in numbers? J Immunother. 2012; 35: 116-124.
- Haymaker CL, Wu RC, Ritthipichai K, Bernatchez C, Forget MA, Chen JQ, et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology. 2015; 4: 1014246.
- Feng Z, Puri S, Moudgil T, Wood W, Hoyt CC, Wang C, et al. Multispectral imaging of formalin-fixed tissue predicts ability to generate tumor-infiltrating lymphocytes from melanoma. Journal for immunotherapy of cancer. 2015; 3: 47.
- Johnson DB, Lovly CM, Flavin M, Panageas KS, Ayers GD, Zhao Z, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res. 2015; 3: 288-295.
- Mangana J, Cheng PF, Schindler K, Weide B, Held U, Frauchiger AL, et al. Analysis of BRAF and NRAS Mutation Status in Advanced Melanoma Patients Treated with Anti-CTLA-4 Antibodies: Association with Overall Survival? PLoS One. 2015; 10: e0139438.
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016; 152: 45-51.
- Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015; 151: 1206-1212.
- Bostwick AD, Salama AK, Hanks BA. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. Journal for immunotherapy of cancer. 2015; 3: 19.
- Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015; 33: 773-781.
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015; 350: 207-211.
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014; 371: 2189-2199.
- Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR American journal of roentgenology. 2011; 197: 992-1000.
- Wolchok JD, Weber JS, Hamid O, Lebbe C, Maio M, Schadendorf D, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010; 10: 9.
- Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. Journal for immunotherapy of cancer. 2015; 3: 23.
- Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011; 108: 16723-16728.
- Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer immunology, immunotherapy : CII. 2014; 63: 247-257.
- Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015; 21: 5453-5459.
- Kitano S, Postow M, Cortez C, Rasalan TS, Gallardo HF, Panageas KS, et al. Myeloid-derived suppressor cell quantity prior to treatment with ipilimumab at 10mg/kg to predict for overall survival in patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012; 30.
- Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015; 25: 208-224.
- Valpione S, Martinoli C, Fava P, Mocellin S, Campana LG, Quaglino P, et al. Personalised medicine: Development and external validation of a prognostic model for metastatic melanoma patients treated with ipilimumab. Eur J Cancer. 2015; 51: 2086-2094.
- Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer immunology, immunotherapy : CII. 2014; 63: 449-458.
- Wilgenhof S, Du Four S, Vandenbroucke F, Everaert H, Salmon I, Lienard D, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013; 36: 215-222.
- Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013; 24: 1697-1703.
- Yuan J, Zhou J, Dong Z, Tandon S, Kuk D, Panageas KS, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2014; 2: 127-132.
- Cipponi A, Wieers G, van Baren N, Coulie PG. Tumor-infiltrating lymphocytes: apparently good for melanoma patients. But why? Cancer immunol immunother. 2011; 60: 1153-1160.
- Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014; 9: e87705.
- Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012; 30: 1835-1841.
- Zelba H, Weide B, Martens A, Derhovanessian E, Bailur JK, Kyzirakos C, Pflugfelder A. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by melan-A but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin Cancer Res. 2014; 20: 4390-4399.
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011; 9: 204.
- Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer immunol immunotherap : CII. 2012; 61: 1019-1031.
- Schindler K, Harmankaya K, Postow M, Frantal S, Bello D, Ariyan CE, et al. Pretreatment levels of absolute and relative eosinophil count to improve overall survival (OS) in patients with metastatic melanoma under treatment with ipilimumab, an anti CTLA-4 antibody. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013; 31.
- Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015; 112: 1904-1910.
- Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, et al. Neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2015; 174: 146-151.
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007; 13: 6681-6688.
- Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008; 26: 5950-5956.
- Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010; 116: 1767-1775.
- Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009; 58: 823-830.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Current oncology (Toronto, Ont). 2013; 20:165-169.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014; 20: 5064-5074.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515: 568-571.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015; 372: 2509-2520.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348: 124-128.
- Campesato LF, Barroso-Sousa R, Jimenez L, Correa BR, Sabbaga J, Hoff PM, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015; 6: 34221-34227.
- Tsutsumi M, Asai J, Wada M, Takenaka H, Katoh N. Malignant melanoma showing a rapid response to nivolumab. Australas J Dermatol. 2016; 57: 61-63.
- Dillman RO, Fogel GB, Cornforth AN, Selvan SR, Schiltz PM, DePriest C. Features associated with survival in metastatic melanoma patients treated with patient-specific dendritic cell vaccines. Cancer Biother Radiopharm. 2011; 26: 407-415.
- Lowe DB, Bose A, Taylor JL, Tawbi H, Lin Y, Kirkwood JM, et al. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology. 2014; 3: e27589.
- Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010; 16: 399-403.
- Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013; 31: 2388-2395.
- Gajewski TF, Zha Y, Thurner B, Schuler G. Association of gene expression profile in metastatic melanoma and survival to a dendritic cell-based vaccine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009; 27.
- Marten A, Sievers E, Albers P, Muller S, Franchy C, von Ruecker A, et al. Telomerase-pulsed dendritic cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Ger Med Sci. 2006.
- Hodi FS, Sznol M, Kluger H, McDermott D, Carvajal RD, Lawrence DP, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-, BMS-936558, ONO-4538) in a phase I trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014; 32.
- Markowicz S, Nowecki ZI, Rutkowski P, Lipkowski AW, Biernacka M, Jakubowska-Mucka A, et al. Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Med Oncol. 2012; 29: 2966-2977.
- Bol KF, Aarntzen EHJG, in 't Hout FEM, Schreibelt G, Creemers JHA, Joost Lesterhuis W, et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. OncoImmunology. 2015.
- Weintraub A. UPDATED: Merck's melanoma 'game-changer' Keytruda likely to bolster drug pricing debate FiercePharma 2014.
- Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013; 31: 999-1008.