
Review Article
Austin J Dermatolog. 2016; 3(3): 1053.
Dermatologic Manifestations of Obesity - Part 2 Endocrine Abnormalities
Reid A Waldman¹* and Anne H Kettler²
¹UMKC School of Medicine, UMKC Vision Research Center, USA
²Department of Dermatology, College Park Family Care Center, USA
*Corresponding author: Reid Alexander Waldman, Fifth Year Medical Student, UMKC School of Medicine, UMKC Vision Research Center, Shawnee Mission, KS, Kansas City, Missouri, USA
Received: July 07, 2015; Accepted: May 19, 2016; Published: May 21, 2016
Abstract
Over the past several decades, the proportion of Americans suffering from obesity has risen drastically. Accompanying this increase in obesity is a concomitant increase in many of the co-morbidities associated with obesity, many of which are seen less frequently in persons with normal body weight. Notably, there has been an increase in dermatologic conditions seen in this special patient population. The skin maladies seen with increased frequency in obese patients are caused by a variety of factors, specifically: (1) the mechanical changes associated with increased weight; (2) the hyperandrogenism of obesity; and (3) the secondary hyperinsulinemia of obesity. Endocrine abnormalities that often accompany obesity and the resultant common dermatologic conditions associated with these abnormalities include acrochordons, acanthosis nigricans, keratosis pilaris, hidradenitis suppurativa, acne keloidalisnuchae, hirsutism, and hypothyroidism.
Keywords: Obesity; Acrochordons; Hyperandrogenism; Hyperinsulinemia; Acanthosis nigricans; Keratosis pilaris; Hidradenitis suppurativa; Acne keloidalisnuchae; Hirsutism; Hypothyroidism
Introduction
This two-part series of articles will review clinical dermatologic manifestations of obesity, identify clinical findings that can serve as harbingers of more serious systemic disease, help direct treatment choices, and ultimately improve patient care outcomes. Part 1 examined the relationship between the mechanical changes caused by obesity and the resultant common dermatologic conditions associated with the changes. [2] Part 2 will explore the relationship between the endocrine abnormalities that often accompany obesity and the resultant common dermatologic conditions associated with these abnormalities.
Acrochordons (Skin Tags)
Acrochordons, known to lay people as skin tags, are brown or flesh-colored soft papules attached by a stalk which occur in 25% of the general population [2]. There is an increased incidence in both the number and density of acrochordons in the obese with a linear correlation existing between increasing BMI and an increase in the presence and density of acrochordons. Most commonly, acrochordons occur on the face, eyelid, neck, axilla, and groin; however, acrochordons occurring on the extremities, vulva, and a variety of other locations have been reported [3]. The primary complaint of the obese patient with acrochordons is cosmetic although some patients note that the lesions can become painful and irritated secondary to the patient’ clothing or jewelry repeatedly rubbing against them. Whereas non-obese patients are likely to only have several acrochordons, obese patients often have large, dense, groupings of the lesions. Although the clinical diagnosis of acrochordons is relatively straightforward, several other skin lesions including basal cell carcinomas and an accessory auricular tragus may mimic acrochordons. When there is a question regarding the correct diagnosis, biopsy of the lesion should be considered. Histologically, the stalk of acrochordons will reveal numerous dilated capillaries, as well as the presence of varied loose connective tissue [4]. The epidermis of the acrochordons characteristically has a combination of benign appearing acanthosis, hyperkeratosis, and papillomatosis [4]. While acrochordons are usually benign and in general their presence is not a harbinger of more serious underlying conditions, acrochordons can occasionally be associated with serious underlying conditions and therefore it is important to keep these associated diseases in mind. This is especially true when encountering the obese patient presenting with the sudden and rapid development of multiple acrochordons. One condition associated with the rapid development of multiple acrochordons is Gardner Syndrome. Gardner syndrome is a potentially fatal, heritable condition which is a subtype of familial colorectal polyposis. Patients suffering from Garner Syndrome exhibit gastrointestinal adenocarcinomatous polyps, osteoma of the skull and jaw, epidermoid cysts, retinal abnormalities, desmoid tumors of the breast and chest wall, thyroid tumors, abnormalities of dentition, and acrochordons (Table 1).
Gastrointestinal adenocarcinomatous polyps
Osteoma of the jaw
Osteoma of the skull
Epidermoid cysts
Congenital hypertrophy of the retinal pigment epithelium
Desmoid tumors of the breast and chest wall
Supranumerary teeth
Acrochordons
Table 1: Clinical Findings of Garner Syndrome.
Acrochordons have also been associated Birt-Hogg-Dube syndrome, an uncommon heritable disease characterized by the presence of a triad of skin lesions: (1) acrochordons; (2) fibrofolliculoma; and (3) trichodiscoma. It is important to identify this uncommon disease as patients suffering from Birt-Hogg-Dube have a seven times greater risk of developing renal malignancies than the general population [5]. Other diseases associated with acrochordons or acrochordons-like skin lesions include Rabson- Mendenhall Syndrome which is a rare heritable disorder caused by severe insulin resistance and Gorlin-Goltz syndrome which is a rare heritable disorder characterized by the early occurrence of multiple acrochordons-like soft pedunculated basal cell carcinomas. Dermoscopy of acrochordons-like skin lesions associated with Gorlin-Glotz syndrome will reveal isolated blue-gray globules, small blue-gray ovoid nests, and fine elongated telangiectases [6]. It is worth noting that some investigators believe that the association of the increased incidence of acrochordons with obesity, like the increased association of acanthosis nigricans with obesity, is the result of insulin resistance rather than the increase in patient weight itself [7]. This theory seems likely given the fact that in the absence of insulin resistance, there is no increased likelihood of either dermatologic malady regardless of the patient’s weight. Diagnosis of acrochordons is primarily clinical. Acrochordons can be safely treated without any histopathological confirmation unless there is a clinical suspicion of more serious underlying disease as mentioned above. Three main excision modalities can be used to treat acrochordons: (1) cryotherapy; (2) electrodessication: and (3) excision with scissors. Cryotherapy and electrodessication have the benefit that these modalities are associated with decreased incidence of bleeding; however, they have the drawback that healing of the thermal lesion may take several days. Excision of small acrochordons with scissors can quickly be done without local anesthetic unless the lesion is large enough that the clinician believes that the discomfort caused by the excision will exceed the discomfort caused by injection of local anesthetic. It is important to wear gloves during removal of acrochordons by scissor excision as bleeding is common.
Acanthosis nigricans
Acanthosis nigricans is a skin condition characterized by a hyperpigmented, roughened, plaque which is often described as velvety or leathery in appearance (Figure 1). In addition to the cosmetic implications, the patient suffering from acanthosis nigricans may also complain that the affected skin is malodorous. This skin disorder is one of the most common dermatologic conditions seen in the obese, affecting roughly 74% of obese patients [8]. Classically, it is found in the flexural areas such as the nape of the neck, the axilla, and the groin; however, it is also observed on many other surfaces such as the vulva and the areola. It is most commonly seen in Native Americans, African Americans, and Hispanics [8]. It has been recently recognized that acanthosis nigricans is occurring more commonly in children as a result of the epidemic of childhood obesity. In fact, recent studies show that nearly a quarter of elementary school aged children who are above 120% of their ideal body weight are afflicted [9]. Diagnosis can be made on a clinical basis; however, if biopsied, acanthosis nigricans is histologically hallmarked by variable acanthosis, papillomatosis, and hyperkeratosis [8].
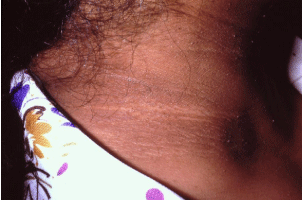
Figure 1: Acanthosis nigricans is a skin condition characterized by a hyperpigmented,
roughened, plaque which is often described as velvety or leathery
in appearance. Classically, it is found in the flexural areas such as the nape
of the neck.
In obese patients, acanthosis nigricans is almost always the result of hyperinsulinemia secondary to tissue insulin resistance. In fact, the relationship between hyperinsulinemia and acanthosis nigricans is so significant that there is a direct correlation between the degree of insulin resistance and the severity of the disease [10]. The reason for this relationship is that excess circulating insulin over stimulates insulin-like growth factor receptors in the skin resulting in significant epidermal proliferation [10]. The resultant hyperkeratosis causes the lesions characteristic hyperpigmentation and velvety appearance. It is worth noting that the hyperpigmentation is not the result of increased melanogenesis and that histologically these lesions do not demonstrate increased presence of melanocytes or melanosomes.
While hyperinsulinemia is one of the most common causes of acanthosis nigricans, it is important for clinicians to be aware of the many other pathological processes that are associated with this common skin disorder. For this reason, the presence of acanthosis nigricans should serve as a warning sign that a serious underlying disease may be present. Physicians should be especially suspicious of these alternative causalities in any patient of normal body weight who develops acanthosis nigricans. It should be remembered that even in the setting of known hyperinsulinemia, a serious systemic disease may co-exist. Other causes of acanthosis nigricans include malignancy (e.g. gastric, colon, liver and ovarian adenocarcinoma), use of nicotinic acid, Cushing’s disease, and hypothyroidism. Additionally, there are a host of other insulin-resistant states that could be responsible for the development of the disease. On rare occasions, familial cases have been documented [11].
While acanthosis nigricans is most often clinically benign, these lesions can cause the patient significant emotional distress and therefore many patients desire cosmetic treatment. Additionally, some patients describe their lesions as malodorous and painful and as a result also desire treatment. Fortunately, there are four groups of treatment modalities available: (1) topicals; (2) oral medications; (3) laser therapy; and (4) surgery. It is the opinion of the authors that all treatment plans should not only address the skin lesions but also should attempt to correct the underlying cause of the lesions.
In the case of obesity-induced insulin resistance, patients who lose weight and become euglycemic will often have improved results ranging from significant improvement to complete resolution of the skin abnormalities. Agents that have been documented to be especially effective in helping treat underlying insulin resistance while the patient is losing weight include metformin and/or thiazolidinediones [12]. Octreotide is also used in patients with insulin resistance as it decreases the secretion of insulin. The clinician should be aware that patients suffering from acanthosis nigricans secondary to insulin resistance will occasionally report a worsening of skin symptomatology upon starting insulin therapy [13]. This phenomenon is especially well documented in patients who fail to rotate injection sites and as a result begin to develop new lesions at these injection sites [13]. In the same way, patients who have acanthosis nigricans as a result of underlying malignancy often have resolution of the skin lesions once resection of the tumor occurs.
In patients in which treatment of the underlying condition provides suboptimal cosmetic results or in those patients in which the underlying condition is unaddressable, topical treatments can be tried. Often times 0.1% tretinoin is successful at lightening the hyperpigmented lesions. There is evidence that combining tretinoin with ammonium lactate 12% is associated with greater improvement than treatment with tretinoinalone [12]. It is worth noting that in cases of vulvar and areolar acanthosis nigricans, irritation from tretinoin use is a common side effect. In this setting, patients can be started with application of 0.025% tretinoin cream every other day with slow elevation to daily use and the increasing of concentrations as side effects allow.
In patients who fail tretinoin therapy, topical calcipitriol therapy has been shown to be effective. Calcipotriol is believed to activate vitamin D3 receptors thus decreasing proliferation of keratinocytes ultimately resulting in lighter lesions [14]. In patients who fail topical therapy, systemic therapy is an option. Oral isotretinoin at an initial daily dose of 50mg has been successfully used to treat acanthosis nigricans. While resolution of the skin lesions is often achieved while the patient is taking this drug, the clinical improvement usually reverses upon discontinuation of the therapy, so most patients will require a daily 25mg dose for maintenance of improvement [15]. For patients in whom medical management fails, dermabrasion, longpulsed laser therapy or surgical excisions are reasonable next options.
Keratosis pilaris
Keratosis pilaris is a skin condition characterized by palpable sterile papules and/or pustules which affects approximately 16% of the population (Figure 2) [16]. It most commonly occurs on the posterior aspect of the upper arms, anterior thighs, face, and buttocks; however, the lesions of keratosis pilaris can occur anywhere except for the soles and palms. While keratosis pilaris is a benign condition, these lesions are associated with significant cosmetic comorbidity with 69% of patients reporting distress as a result of their rash [16]. This is especially worrisome considering these lesions most commonly occur in adolescent females, many of whom may already be experiencing associated body image issues. Patients who are most likely to experience considerable distress from this condition are those with facial involvement, those with significantly inflamed, red lesions, and those who are already suffering from poor body image.
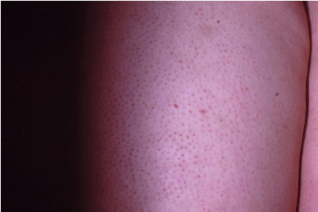
Figure 2: Keratosis pilaris is a skin condition characterized by palpable sterile
papules and/or pustules which affects approximately 16% of the population.
It most commonly occurs on the posterior aspect of the upper arms, anterior
thighs, face, and buttocks.
While the etiology of keratosis pilaris is unknown, there are a number of conditions with which this skin disorder has been linked. Most notably, keratosis pilaris is associated with obesity, dry skin, and atopic diathesis [17]. As a result of its association with dry skin, this condition often flares in the winter. It is worth noting that its association with obesity is believed to be caused by hyperinsulinemia. It is thought that the increase in circulating insulin triggers associated increases in androgen production leading to follicular keratinization resulting in the development of keratosis pilaris [17]. This physiologic change is of clinical importance as insulin-dependent patients may develop lesions at their insulin injection sites. The diagnosis of keratosis pilaris is generally made on clinical grounds; however, it is important that clinicians differentiate keratosis pilaris from other, more serious lesions. Other conditions that are often confused with keratosis pilaris include acne vulgaris, heat rash, and bacterial folliculitis. While facial keratosis pilaris can appear similar to acne, keratosis pilaris can be differentiated from acne due to the presence of uniform lesions, the absence of comedones, and its occurrence in the setting of cracked, dry skin. Additionally, the presence of a reddish halo surrounds the lesions and the histologic finding of inflammation exclusively in the area surrounding the follicle, but not invading the follicle itself, can be considered diagnostic for keratosis pilaris [16]. Finally, while keratosis pilaris is a distinct entity from bacterial folliculitis, the lesions of keratosis pilaris can become secondarily infected. This occurs primarily when the patient scratches or picks at lesions. Secondary infection of keratosis pilaris should be considered in any patient with previously localized, stable keratosis pilaris who develops sudden, widespread proliferation of new lesions in patients. These patients should be treated with oral antibiotics that are effective against Streptococcus Pyogenes and Staphylococcus Aureus.
In patients who desire cosmetic improvement of keratosis pilaris, there are a number of treatment options available; however, response varies significantly on a patient-to-patient basis. Furthermore, few studies have examined the efficacy of the myriad of treatment modalities recommended for this disease. It is the opinion of the authors that all patients suffering from keratosis pilaris should be offered treatment due to evidence that the vast majority of patients suffering from this skin disorder experience emotional distress secondary to the effect on appearance.
Since keratosis pilaris often spontaneously resolves in many patients after adolescence, reasonable first step in the treatment of patients with mild disease is the use of keratolyic emollient creams (e.g. combination urea/salicylic acid products and ammonium lactate). The use of these products is primarily successful at decreasing the roughness of the skin lesions. For patients who respond to emollients, but desire further treatment, there is recent evidence that the addition of a sonic skin care brush can provide additional improvement [18]. Patients should be informed that overuse of these products can actually worsen keratosis pilaris by causing further skin dryness which exacerbates the lesions. In patients with more diffuse keratosis pilaris or for those who fail emollient therapy, both 0.05% tazarotene and 0.1% tacrolimus have been found to be effective. In fact, a recent study demonstrated that 80% of patients treated with 0.1% tacrolimus reported at least 75% improvement [19]. Finally, in patients experiencing an acute flair of keratosis pilaris or those who desire relief of the redness associated with the disease for a special occasion, the temporary use of triamcinolone cream is warranted.
Hidradenitis suppurativa
Hidradenitis suppurativa is a common, yet underdiagnosed dermatologic condition characterized by recurrent painful, malodorous nodules found primarily in the areas of skin which contain the apocrine sweat glands (Figure 3). While the exact etiology of this disease is unknown, research has identified abnormalities of the hypothalamopitutiary axis in many patients suffering this disease [20]. These lesions are often associated with significant abscess formation with the possibility of sinus tract formation and significant scarring. Most commonly, this condition presents in the groin, axilla, buttocks, and inframammary region; however, lesions have been identified in areas not typically thought to possess apocrine sweat glands. The typical patient affected by hidradenitis suppurativa is a post-pubertal, overweight smoker with a 3:1 ratio of female: male patients afflicted [21].
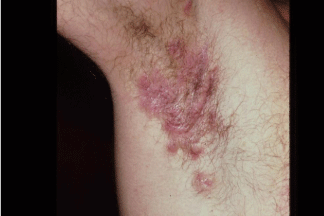
Figure 3: Hidradenitis suppurativa is a common, yet underdiagnosed
dermatologic condition characterized by recurrent painful, malodorous
nodules found primarily in the areas of skin which contain the apocrine sweat
glands.
The diagnosis of hidradenitis suppurativa is made on clinical grounds. The disease may present in three clinical stages. Stage 1 is characterized by the presence of one or more nodules in the absence of sinus tract formation or scarring. Because there are no pathognomonic features of these nodules, they are often misdiagnosed as abscesses or boils. This misdiagnosis is often ‘supported’ by the fact that early episodes of hidradenitis suppurativa tend to spontaneously resolve within 10 days [31]. The astute clinician should consider the diagnosis of hidradenitis suppurativa in any patient with nodules found in the groin, axilla, buttocks, and inframammary region. Additionally, it is possible to further distinguish the nodules of hidradenitis suppurativa from boils based on the fact that while boils have a typically pointed shape, the lesions associated with hidradenitis suppurativa are rounded and lack any central necrosis (Figure 4). Due to low index of suspicion for the disease coupled with the fact that early episodes resolve spontaneously, hidradenitis suppurativa, is often not diagnosed until the disease progresses to Stage 2 [22]. Patients suffering from Stage 2 hidradenitis suppurativa will exhibit multiple, recurrent nodules with associated sinus tract formation and scarring. It is worth noting that patients often have intermittent foul smelling discharge from the sinus tracts and that this is a cause of embarrassment as it may cause them to have to place pads or diapers on the affected areas. This is typically the stage at which hidradenitis suppurativa is diagnosed which is astounding as the average patient reports having had flares for an average of 12 years at the time of diagnosis [22]. By the time the disease has progressed to Stage 2, significant disfigurement and emotional distress is present, with many patients developing depression as a result. 99% of patients do not have disease progression beyond Stage 2; however, for the rare patient whose disease progresses to Stage 3, the results can be devastating [23,24]. Stage 3 is characterized by near complete involvement of the affected areas with rampant scarring and multiple interconnected sinus tract formation. These patients often experience significant pain and many suffer from significant discharge from the sinus tracts. In severe cases, dermal contractions and rope-like elevation of the skin may occur. Finally, regardless of what stage of disease a patient presents with, there are three crucial considerations to keep in mind. First, biopsy of the affected area is not warranted as there is no characteristic histologic appearance of the condition [35]. Second, while the condition looks very similar to boils and abscesses, there is usually not a primary infectious component of the disease, therefore, there is no need culture the lesions unless there is a strong belief that there is a secondary infection. Third, the condition can appear very similar to a number of other very different diseases including actinomyces infection, nodular acne, Bartholin’sGlandcysts, inflamed and/or infected epidermoid cysts, pilonidal cysts, and lymphogranuloma venereum. When managing a patient with hidradenitis suppurativa, it is important to recognize that there are a number of possible complications and associated conditions that should be considered throughout the course of the disease. Most often the clinical course is complicated by the development of super infections and lymphatic obstruction with associated lymphedema. Marjolin’s ulcers and fistula formation into internal organs (e.g. bladder, intestine, ureters etc.) occur less commonly. Additionally, patients with hidradenitis suppurativa are more likely than the unaffected population to have intractable acne, Crohn’s disease, amyloidosis, arthritis, as well as a number of other conditions [23]. Severe depression is common.
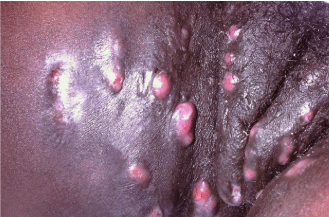
Figure 4: It is possible to distinguish the nodules of hidradenitis suppurativa
from boils based on the fact that while boils have a typically pointed shape,
the lesions associated with hidradenitis suppurativa are rounded and lack any
central necrosis. Hidradenitis Suppurativa is shown here.
Treatment of hidradenitis suppurativa is dependent on the clinical stage a patient presents with. Unfortunately, even with the best treatment plans, treatment results are often suboptimal. There are very few trials evaluating many of the available treatment modalities; however, expert opinion exists supporting a number of options. For patients with Stage 1 disease, treatment with topical clindamycin is a reasonable first step. It is important to advise patients undergoing topical treatment that resolution of nodules may take more than 3 months [26]. In patients with especially painful lesions or with acute flairs, intralesional injections of triamcinolone as well as oral steroid bursts have been utilized with reported success. Some authors advocate the use of limited surgical excision at this stage despite the high rate of reoccurrence that occur despite surgical treatment [26].
In patients with more advanced disease a number of other treatment options exist. Most often, oral tetracycline is started for its anti-inflammatory effect; however, there is conflicted evidence over whether this is more effective than topical antibiotics [36]. Recently evidence has emerged indicating that TNF-alpha inhibitors such as infliximab are effective in treatment, but while many patients achieve early gains, sustained remission only occurs in 60% of patients at 18 months [27]. In patients who are refractory to treatment with TNFalpha inhibitors and who do not want to pursue surgical options, treatment with cyclosporine is a reasonable next step, although relapse after discontinuation of cyclosporine often occurs. Finally, in patients with advanced disease or with significant quality of life impairment who are refractory to medical management two additional invasive options exist: (1) surgical excision; and (2) laser therapy. In patients who are undergoing surgical management, recent recommendations advise total excision of the apocrine gland bearing region surrounding the lesions as excisions of just the lesions themselves will often result in recurrence. Additionally, ablation of the affected area with CO2, neodymium-doped yttrium aluminium garnetlasers may be effective in selected patients.
Acne keloidalis nuchae
Another dermatologic condition that is seen with greater incidence in the obese patient with insulin resistance is acne keloidis nuchae. Acne keloidalis nuchae affects the occiput and scalp and occurs in the setting of follicular inflammation. Often the inciting event can be linked to follicular injury during a close haircut or razor shave of the neck. Typically, patients suffering from acne keloidalis nuchae present initially with pruritic papules and pustules arising from the hair follicles of the nape of the neck. This results in chronic inflammation and folliculitis which eventually causes the development of hard, hypertrophic nodules and scarring. This scarring can enlarge to form keloid-like bands along the hairline. Once the chronic form of the disease develops, the affected scarred areas undergo irreversible hair loss which is often psychologically distressing to the patient, causing them to seek medical attention. Acne keloidalis nuchae affects men 20 times more commonly than women [28]. The typical patient with this condition is a young, Afro-Caribbean male with black curly hair. Common risk factors include specific hair types and styles, frequent helmet wearing, frequent haircuts, high tight collars, insulin resistance and obesity. In fact, recent evidence has shown that the relationship between metabolic syndrome and acne keloidalis nuchae is so unique that some patients develop the disease without any other risk factors [29]. Despite the name and similar appearance, this condition is neither related to acne vulgaris nor keloid formation. Diagnosis of this condition is primarily clinical; however, it can be differentiated from acne vulgaris, hidradenitis suppurativa, folliculitis decal vans, and other scarring alopecias based on its unique histologic appearance on biopsy. Histologically, lesions of acne keloidis nuchae are characterized by granulomatous inflammation with the presence of both neutrophils and lymphocytes invading the lower isthmus of the hair follicle (Figure 5) [30]. Additionally, sebaceous gland destruction and hypertrophic scarring are present. Histologically, the presence of hypertrophic scarring is what primarily helps distinguish acne keloidalis nuchae from folliculitis decal vans. Due to the significant quality of life concerns associated with the development of acne keloidalis nuchae, many patients wish to be treated. All treatment strategies should begin with education about minimizing risk factors including promoting avoidance of further mechanical trauma to the skin of the neck during haircuts and shaving and weight loss if obesity is present. From there, treatment choices should be based on how many papules are present, whether pustules are present, and whether hypertrophic scars have formed. In patients with early papular disease, treatment primarily consists of clobetasol propionate foam combined with intralesional 5mg/ cc triamcinolone injections for larger lesions. In patients who have suboptimal responses to topical steroids, a next logical step is the use of topical immune modifiers pimecrolimus or imiquimod [31]. Prevention of further papule formation can be fostered by having patients wash with alpha-hydroxy acid, chlorhexidine, or benzoyl peroxide. In patients who have pustule formation, these lesions should be cultured and appropriate antibiotic therapy should be used. Often tetracycline 500mg BID is used for both its antibiotic activity and anti-inflammatory activity. In patients with large hypertrophic scars, recurrence of acne keloidalis nuchae is common. In this setting, intralesional corticosteroid injections are a reasonable first step. In patients who do not respond to steroid injections, surgical excision or electrodessication can be attempted. Recent evidence shows that patients who undergo excision have better outcomes if excision sites are allowed to undergo secondary intention healing [32]. Additionally, while some authorities recommend the use of cryotherapy, it is the opinion of the authors that this treatment modality should not be used in dark skinned patients with Fitzpatrick Skin Types IV, V, and VI, as these patients often experience hypo pigmentation which can last up to 18 months. Finally, recent studies have focused on the use of laser therapy on hypertrophic lesions. Impressively, one such study found that patients who underwent four rounds of diode laser therapy displayed on average 90-95% improvement with no new lesion development within the six months following therapy [33]. Similar efficacy has been found when patients are treated with neodymiumdoped yttrium aluminium garnet and CO2 lasers.
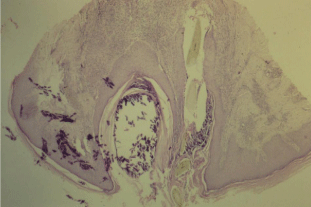
Figure 5: Histologically, lesions of acne keloidis nuchae are characterized
by granulomatous inflammation with the presence of both neutrophils and
lymphocytes invading the lower isthmus of the hair follicle.
Hirsutism
Hirsutism is defined as the development of excess androgendependent hair in women. While many discount hirsutism as solely a cosmetic issue, its detrimental effect on female body image cannot be underestimated, making it a significant concern tohealthcare provider. While there are many causes of hirsutism that will be discussed later in this section, its association with obesity is especially problematic. Hirsutism occurs in obese patients for two reasons: (1) the presence of hyperinsulinemia in obese women leads to the increased production of ovarian androgens; and (2) the increased quantity of adipose tissue in these patients leads to greater testosterone production [34]. The reason hyperandrogenism results in hirsutism is that in some areas of the body (e.g. the lips, chin, chest, and sideburns) androgens promote an increase in follicle size, the conversion of vellus hairs to terminal hairs, and an increase in length of the anagen phase of hair growth [34]. Due to the fact that hirsutism can serve as a harbinger of more serious systemic disease, patients presenting with this condition should undergo a work-up to identify potentially dangerous underlying causes. Possible etiologies of hirsutism include: obesity, polycystic ovarian syndrome, certain drugs, idiopathic causes, and neoplasms. Patients with the rapid onset of hirsutism, development of hirsutism in the pre- or post-pubertal period, severe hirsutism, multiple signs of virilization, or with multiple signs and symptoms of Cushing’s disease should undergo further testing (Table 2). Laboratory testing should include testosterone levels, DHEAS, LH, FSH, and Cortisol and ACTH determination if suspicious of the diagnosis of Cushing’s disease. Many patients with hirsutism will have normal levels of testosterone because their hirsutism is a result of increased follicular sensitization to testosterone rather than increased circulating testosterone levels [25].
Weight gain
Moon facies
Buffalo hump
Easy bruisability
Acne
Slow skin healing
Fatigue
Muscle weakness
Glucose intolerance
Emotional lability
Erectile dysfunction
Decreased libido in men
Decreased fertility in men
Hirsutism in females
Oligomenorrhea
Table 2: Signs and Symptoms of Cushing Syndrome.
Treatment of hirsutism involves a multi-faceted approach and patience. In the interim between the start of treatment and when results are expected, patients can be encouraged to undergo physical epilation by shaving and waxing to provide immediate body image improvement. In patients with hirsutism secondary to obesity, weight loss should be encouraged and insulin sensitizing agents such as metformin and thiazolidiones can be used; however, there is conflicting evidence on whether insulin sensitizers create meaningful improvement in hirsutism [25].
In general, a first-line strategy in the medication management of should consist of a combination of oral contraceptives (estrogenprogesterone combination) and spironolactone. Oral contraceptives are effective at decreasing hirsutism because they: (1) increase circulating levels of sex hormone binding globulin thus decreasing the levels of free testosterone; and (2) because they decrease ovarian androgen synthesis. These effects are magnified by the use of spironolactone which blocks androgen activity at receptor sites. In patients who are not candidates for spironolactone (e.g. renal failure, hyperkalemia, etc.) finasteride is another medication which can be used in combination with oral contraceptives. Finasteride inhibits 5-alpha reductase thus decreasing the conversion of testosterone to the more potent dihydrotestosterone. The disadvantage of finasteride is that it takes longer to achieve meaningful improvement than spironolactone. Both spironolactone and finasteride should always be used in conjunction with oral contraceptives in women of childbearing age as these medications can feminize male fetuses if taken during pregnancy [36]. In patients who are either unamenable to, or fail medical management, laser therapy is a next logical step. It is the opinion of the authors that laser therapy should not be used in Fitzpatrick Skin Types IV-VI due to the significant risk of hyperpigmentation. There are many types of laser therapy available that have long-term efficacy; however, all types require multiple treatments and therefore can be very expensive.
Hypothyroidism
Changes in body weight and composition often accompany thyroid disease, with central obesity, weight gain, decreased thermo genesis, and decreased metabolic rate most commonly associated with hypothyroidism. In addition to these changes, skin, hair, and nail changes are also associated with hypothyroidism. Thickening and dryness of the skin, decreased scalp, eyebrow, and body hair, and thin, brittle nails are commonly observed. While diagnosis of hypothyroidism can often be made on the basis of dermatologic signs and symptoms, levels of free T3, T4, and thyroid stimulating hormone should be measured not only to confirm the diagnosis but to provide a baseline for treatment.
Conclusion
This article highlights the magnitude of the effect obesity has on the skin. Not only does the mechanical force created by the obese patient’s extra weight cause a host of conditions, but the endocrinologic abnormalities associated with obesity can cause a variety of other skin diseases. The effect obesity has on the skin is so significant that many of the aforementioned conditions can occur in patients without any other identifiable risk factors. This fact alone should cause clinicians to consider Body Mass Index (BMI) evaluation as a crucial component of any dermatologic examination.
References
- Glauser TA, Roepke N, Stevenin B, Dubois AM, Ahn SM. Physician knowledge about and perceptions of obesity management. Obes Res Clin Pract. 2015; 9: 573-583.
- Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Acrochordon. Am Fam Physician. 2012; 85: 1191-1192.
- Kassinove A, Raam A. Acrochordon of the labia. The Journal of Emergency Medicine. 2013; 44: 361-362.
- Ilango N, Jacob J, Gupta AK, Choudhrie L. Acrochordon-a rare giant variant. Dermatol Surg. 2009; 35: 1804-1805.
- Vincent A, Farley M, Chan E, James WD. Birt-Hogg-Dubé syndrome: a review of the literature and the differential diagnosis of firm facial papules. J Am Acad Dermatol. 2003; 49: 698-705.
- Feito-RodrÖguez M, Sendagorta-CudÃs E, Moratinos-MartÖnez M, GonzÃlez-Beato MJ, de Lucas-Laguna R, Pizarro A. Dermatoscopic characteristics of acrochordon-like basal cell carcinomas in Gorlin-Goltz syndrome. Journal of the American Academy of Dermatology. 2009; 60: 857-861.
- Bhargava P, Mathur SK, Mathur DK, Malpani S, Goel S, Agarwal US, et al. Acrochordon, diabetes and associations. Indian J Dermatol Venereol Leprol. 1996; 62: 226-228.
- Kapoor S. Diagnosis and treatment of Acanthosis nigricans. Skinmed. 2010; 8: 161-164.
- Guran T, Turan S, Akcay T, Bereket A. Significance of acanthosis nigricans in childhood obesity. J Paediatr Child Health. 2008; 44: 338-341.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. Journal of investigative Dermatology. 1992; 98: 82-85.
- Taylor G, James MP, Simpson H. Familial acanthosis nigricans. J R Soc Med. 1994; 87: 169.
- T Isken T, Sen C, Iscen D, Yildiz K. Acanthosis Nigricans and an alternative for its surgical therapy. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2009; 62: 148-150.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011; 94: 34-36.
- Lee HW, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Hyperkeratosis of the nipple associated with acanthosis nigricans: treatment with topical calcipotriol. J Am Acad Dermatol. 2005; 52: 529-530.
- Eberting CL, Javor E, Gorden P, Turner ML, Cowen EW. Insulin resistance, acanthosis nigricans, and hypertriglyceridemia. J Am Acad Dermatol. 2005; 52: 341-344.
- Ahlawat D. Keratosis pilaris. Indian Pediatr. 2002; 39: 1165-1166.
- Yosipovitch G, Mevorah B, Mashiach J, Chan YH, David M. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000; 201: 34-36.
- Kearney M, Henes E, Ortblad K. Efficacy and safety of a sonic skin care brush on keratosis pilaris. Journal of the American Academy of Dermatology. 2013; 68: 49-50.
- Breithaupt AD, Alio A, Friedlander SF. A Clinical study to evaluate the efficacy of tacrolimus 0.1% for the treatment of keratosis pilaris. Journal of the American Academy of Dermatology. 2011; 28: 459-460.
- Harrison BJ, Read GF, Hughes LE. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br J Surg. 1988; 75: 972-975.
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009; 23: 985-998.
- Fialova J, Hercogov J. Hidradenitis Suppurativa. Journal of the American Academy of Dermatology. 2012; 66: AB16.
- Buimer MG, Wobbes T, Klinkenbij JH. Hidradenitis Suppurativa. Br J Surg. 2009; 96: 350-360.
- Gönül M. Hidradenitis Suppurativa. Türk dermatoloji dergisi. 2009; 3: 9-12.
- Shah N. Hidradenitis suppurativa: a treatment challenge. Am Fam Physician. 2005; 72: 1547-1552.
- Pilar M, Coruna, Fonseca E, Verea MM. Infliximab treatment for hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2007; 66: 16.
- Al-Zaid T, Vanderweil S, Zembowicz A, Lyle S. Sebaceous gland loss and inflammation in scarring alopecia: a potential role in pathogenesis. J Am Acad Dermatol. 2011; 65: 597-603.
- Akaberi A, Paricher K, Noorbais MT. Acne keloidalis nuchae in a Caucasian Woman. Journal of Pakistan Association of Dermatologists 2011; 21: 66-68.
- Al-Ajam A, Seymour A, Mooty M. Use of imiquimod and pimecrolimus cream in the treatment of acne keloidalis nuchae. Journal of the American Academy of Dermatology. 2005; 52: 64.
- Bajaj V, Langtry JA. Surgical excision of acne keloidalis nuchae with secondary intention healing. Clin Exp Dermatol. 2008; 33: 53-55.
- Shah GK. Efficacy of diode laser for treating acne keloidalis nuchae. Indian J Dermatol Venereol Leprol. 2005; 71: 31-34.
- Kalu E, Gilling-Smith C. Hirsutism, Obstetrics, Gynaecology & Reproductive Medicine. 2008; 18: 115-119.
- Shah D, Patel S. Hirsutism. Gynecol Endocrinol. 2009; 25: 140-148.
- Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Insulin sensitizers for the treatment of hirsutism: a systematic review and meta-analyses of randomized controlled trials. The Journal of Clinical Endocrinology & Metabolism. 2008; 93: 1135-1142.
- Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010; 316: 165-171.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006; 24: 247-255.